Tiny Inclusions Reveal Diamond Age and Earth’s History: Research at the Carnegie Institution
January 31, 2014

We’ve traveled to the Carnegie Institution of Washington—more specifically the Institution’s Department of Terrestrial Magnetism—to document Dr. Shirey’s work on determining the age of diamonds from many mines around the world by analyzing the tiny mineral inclusions preserved within them.

Dr. Steven Shirey, a senior scientist at the Carnegie Institution of Washington’s Department of Terrestrial Magnetism, has a special interest in diamond—it’s his best method of glimpsing the unimaginable pressures and temperatures of the deep earth’s interior. Duncan Pay © GIA, courtesy Carnegie Institution of Washington
“Let me try to place into a broader context what the good of this diamond work is,” Shirey begins. “One of my personal goals is to take the knowledge of the age of the diamond, and the initial ratios we get from these inclusion studies and put them into the big geological picture in a systematic way. I want to look at how diamonds are distributed across continental terranes with regard to how the continent was put together.”“I want to go back in Earth’s history to see if the pattern of diamond growth and diamond history tells us anything unique about the evolution of Earth as a planet,” Shirey says. “In itself that’s a pretty amazing concept. We’re taking these tiny grains—very, very small—and using the systematic change in chemistry to say something about our entire planet.”
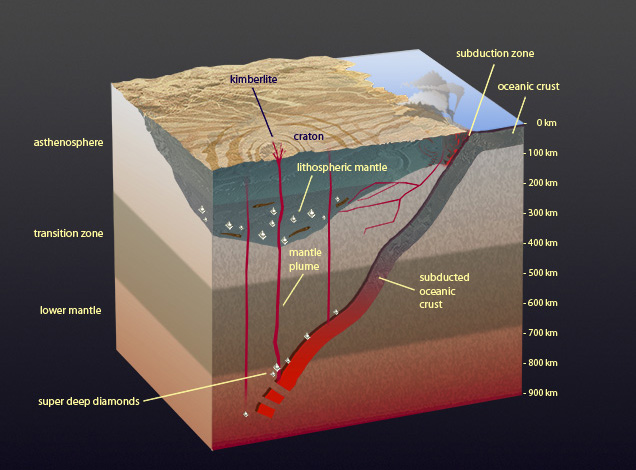
In ancient continental areas, there’s a significant keel, or downward protrusion into the mantle, where conditions are stable enough to preserve diamonds for many hundreds of millions of years. Recent discoveries show that diamonds might form beneath subduction zones at great depths, and that very rarely plumes in the earth’s mantle move them up to where kimberlites can bring them to the earth’s surface. These “superdeep” diamonds provide Shirey and other scientists a tantalizing window into the workings of the deeper mantle. Peter Johnston © GIA
This article complements the winter 2013 Gems & Gemology paper “Recent Advances in Understanding the Geology of Diamond,” coauthored by Shirey and GIA’s Dr. Jim Shigley.Shirey is a one of a collaborative group of geoscientists from institutions all over the world using diamonds as a means to sample the deep earth. His particular specialty involves seeking out the rare individual diamonds that contain tiny and specific sulfide inclusions, painstakingly removing the inclusions and analyzing their contents—in this case, trace amounts of the radioactive isotopes of the rare metals osmium (Os) and rhenium (Re)—to determine ages on the order of billions of years.
An isotope of a chemical element is simply an atom with the same atomic number as that element, but with a slightly different mass, resulting from a different number of neutrons in its nucleus. Some isotopes are stable, but others—called radionuclides—emit radiation and decay into different isotopes over long periods of time. If scientists know the rate of radioactive decay, and have instrumentation capable of measuring the different isotopes present, they can calculate the age of an item very accurately.
This is the principle behind long-established techniques such as radiocarbon dating, which has been widely used in archaeology. Diamonds are vastly older than any archeological relic, so carbon dating—which can only date items back to around 60,000 years ago—isn’t possible.
The isotopes Shirey seeks provide a much longer reach back into the earth’s history. These radioactive isotopes are like tiny, slow-ticking clocks captured in the fabric of a diamond crystal. They decay very slowly over eons, with very long half-lives (the time it takes for a substance’s radiation to fall to half its original level). The isotope of rhenium he uses, 187Re, decays into osmium (187Os) very slowly, with a half-life of 41.6 x 109 years, or 41 billion years.
Research using the rhenium-osmium decay system proves that some diamonds are of remarkable antiquity, says Shirey. “They’re sometimes the oldest minerals we can find on the earth…up to 3.5 billion years old, whereas the earth is only 4.5 billion years old, so they’re often three-quarters of the age of the earth.”
Shirey adds that diamonds are also special because they’re the deepest minerals we can obtain as natural samples to study the earth. Unlike any other mineral, diamonds reach the surface from great depths—up to 700 kilometers (435 miles) beneath the earth’s surface—and may retain unaltered inclusions of mantle minerals within them. A diamond crystal is a “really good container” for these exotic minerals, he says, and preserves them in “pristine” form on the way to the earth’s surface.
“So in one mineral species,” he says, “you have the deepest, the oldest, and the most resistant to secondary effects mineral that you can get, so it makes them very, very unique specimens.”
Dating Diamonds
Dr. Shirey explains the study of radiogenic isotopes hidden inside individual diamond inclusions to determine their age and hence the age of the diamonds themselves.
Shirey tells us that the osmium-rhenium radioactive decay system allows researchers to date individual inclusions. Previous dating techniques required researchers to collect inclusions of other minerals—including garnets and zircon—from many different diamonds to obtain an age. If there was more than one generation of diamonds in the sample, you’d end up with an average age rather than the specific age.
This slideshow takes you through the processes Dr. Shirey uses to select natural diamonds for research and extract the sulfide inclusions from them to obtain accurate ages—often in the range of billions of years. It involves ingenious solutions to fashion the tiny diamonds into plates for study, cleave out the sulfides, separate the isotopes of rhenium and osmium by chemistry, and count the various isotopes with sophisticated laboratory instrumentation for the final age determination.
Selecting Diamonds for Study
The process begins with suitable inclusion-bearing diamond crystals. As Shirey tells us, obtaining such diamonds for study is quite a challenge. “We have to go to a mine or some place that’s being very aggressively prospected so that they’re processing large amounts of kimberlite for diamond grade.”Diamonds are trace minerals in the rock—kimberlite, or more rarely a lamproite as in Australia’s Argyle mine—that carried them up from the mantle. “A diamond in a kimberlite occurs at the part-per-billion level,” says Shirey, “so the average person walking around on a kimberlite is not going to find a diamond sitting there—that’s an extremely rare occurrence.”

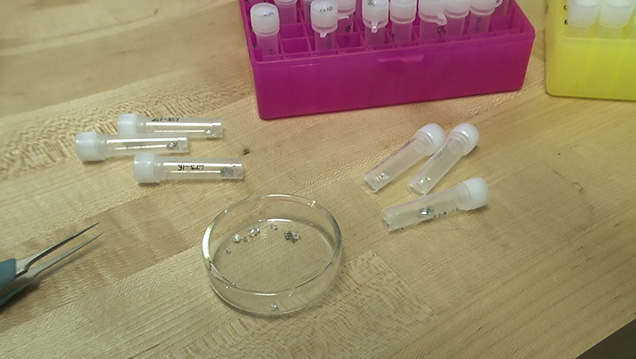
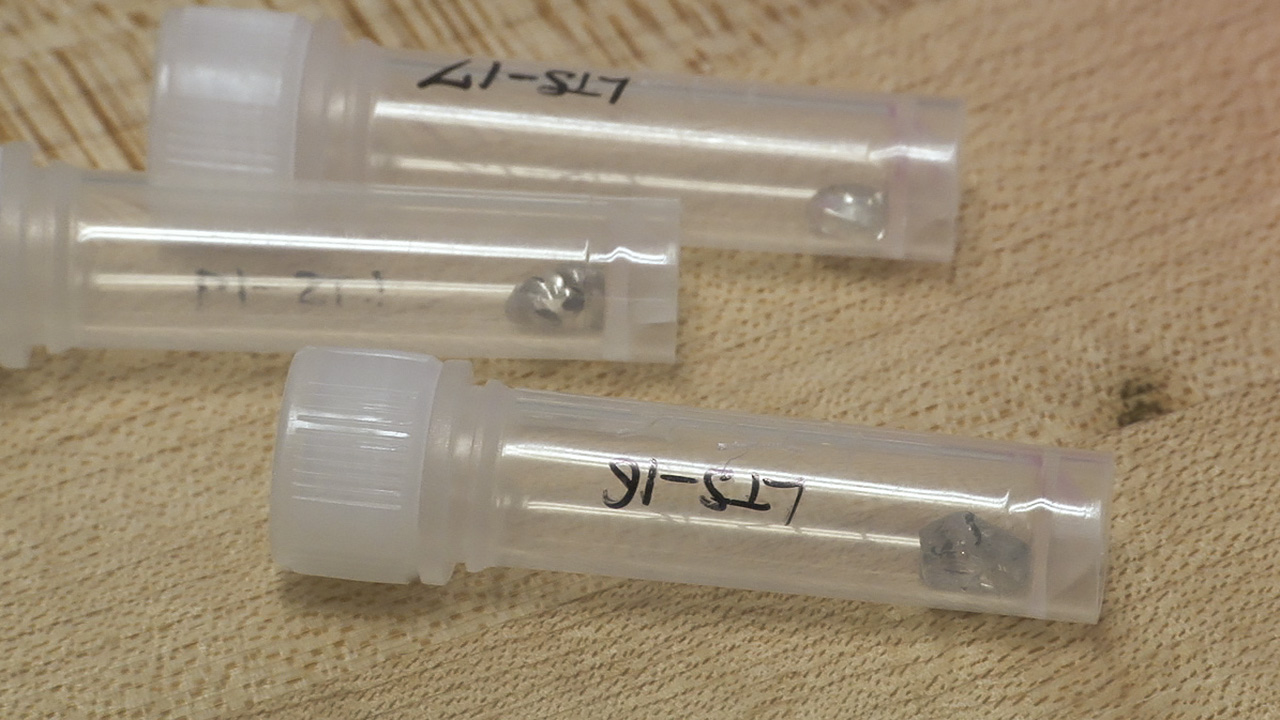
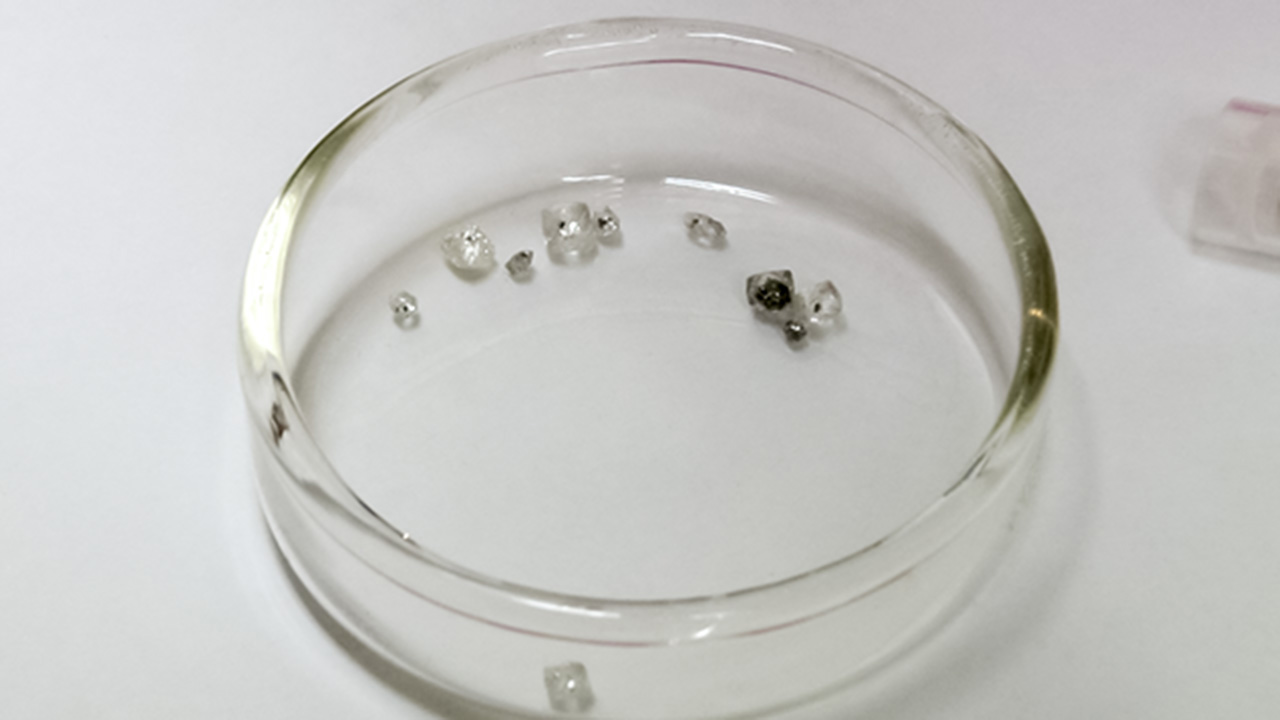
This diamond crystal, from Lesotho’s Letseng mine, contains a sulfide inclusion suitable for Shirey’s research. Pedro Padua © GIA, courtesy Carnegie Institution of Washington
Once researchers have traveled to suitable mining or exploration operations, where large amounts of diamond-bearing ore are produced, they have to pick through the production. Even at a mine as large as South Africa’s Venetia, only 1 in 50,000 diamonds produced might harbor the right kind of inclusion.“In this case we’re seeking the rejects of the gem trade,” says Shirey. “Most mines have a very low frequency of inclusion-bearing diamonds to gem-quality diamonds, and so in some mines it’s almost impossible to get inclusion-bearing stones.”

These diamond crystals, which bear sulfide inclusions decorated by rosette fracture systems, are suitable candidates for dating using the rhenium-osmium decay system. Pedro Padua © GIA, courtesy Carnegie Institution of Washington
Preparing the Diamond for Research
Once the researchers have enough inclusion-bearing diamonds for a valid study, work can begin. “We have to get the inclusion out without breaking it,” says Shirey. “We need to recover the whole inclusion, and we also need to characterize the diamond as fully as possible.“Our job is not just to measure the isotope composition of the inclusion, but also to get the whole picture of how the diamond formed, before we end up destroying the diamond to get the inclusion out.”
The first step is to slice the diamond very precisely across its growth zones using a diamond-cutting laser. “It would probably drive everybody in the gem business crazy to understand that we take a beautiful rough diamond and slice the center out of it,” says Shirey with a wry smile.

Shirey designed this jig for laser cutting the diamond crystals. Duncan Pay © GIA, courtesy Carnegie Institution of Washington
“I actually had to design a device, and I went to the shop and asked if they could make it for me. And they turned it out in a day or two. We brought it back to the laser cutter and set it up, and the guys that owned the laser cutter liked it so much. It’s a little jig that has some thumbscrews that hold the diamond, just in perfect position to cut it—zip, zip, without having to reorient it—and cut three at a time.”The wafer, with the inclusion in the middle of it, is then polished on a conventional diamond scaife. Great care must be taken to ensure the inclusions aren’t compromised or contaminated by the cutting and polishing process.
This yields a precisely oriented polished diamond plate, typically about a millimeter deep, that shows the inclusion’s position relative to the host diamond’s growth zoning. The diamond’s growth zones are especially visible using cathodoluminescence (CL) imaging. CL imaging reveals whether the diamond has a straightforward or more complex growth history.
Shirey’s colleague Dr. Jianhu Wang can also investigate the diamond plate’s isotopic composition using a secondary ion mass spectrometer (SIMS) or ion probe. The SIMS instrument allows detailed chemical analysis of the diamond’s growth zones. It can measure differences between the carbon or nitrogen isotopes across a diamond, which can establish whether the stone has a unique geologic history and what the data from the inclusion means. We will spotlight Dr. Wang’s work in a future Research & News article on the GIA website.
Preparing Diamonds for Study
In this sequence of videos, Dr. Shirey explains how researchers select and prepare suitable inclusion-bearing diamonds for study. The process begins with crystals that are polished into precisely oriented plates for analysis. It ends with the plates being cleaved in a specially designed “diamond cracker” to recover their sulfide inclusions for age determination using chemistry.
Removing the Inclusion from the Diamond
Selecting, documenting, and gathering analytical data on a single diamond plate might take many weeks. From this data, however, researchers gain valuable insight into a diamond’s growth history.At some point, though, the sulfide inclusions have to be removed from the plate for researchers to determine their age. This is the “lowest-tech” point of the process. It’s accomplished using a device Shirey calls the “diamond cracker.” This is a thick-walled hollow steel cylinder with a lid containing a piston. The diamond plate sits over a pair of tungsten carbine lathe bits, which form a platform. Shirey orients the diamond plate within the cylinder so that the portion with the inclusion lies above the gap between the two lathe bits.
On the inside of the cylinder’s lid, there’s a tungsten carbide rod bonded to the surface. Grooves inscribed on the cylinder base and the side of the lid help Shirey align it so that the rod is directly parallel and above the gap between the two tungsten carbide lathe bits in the cylinder base.
Shirey adjusts the piston in the lid so that there’s just enough play to contact the diamond without crushing it. A sharp tap on the top of the piston with a small hammer cleaves the diamond in the plane of the sulfide inclusion, releasing it.
Next, Shirey has to painstakingly search the cylinder’s interior for the inclusion. It’s often tough to find among the shards of diamond and tiny metal fragments from the tungsten carbide components or the steel wall of the diamond cracker. “The inclusion is often 100–300 microns…sometimes it’s 50 microns,” Shirey says. (One centimeter = 10,000 microns, so even a 300-micron inclusion is only 0.03 centimeters across—just 0.3 of a millimeter!)

The tiny reflective speck at the center of the black disk is a sulfide inclusion, which has been cleaved out of a diamond plate using the “diamond cracker.” Hidden inside this tiny speck are traces of the rare elements rhenium and osmium. Pedro Padua © GIA, courtesy Carnegie Institution of Washington
At that size, simply moving the inclusion can be a big challenge: “That’s so small that static forces make it almost impossible to move the inclusion around, and so we have to be very careful not to lose the inclusion,” Shirey continues with a chuckle. “There have been times when I dropped the inclusion in the sink, and I had no recourse but to take the trap off the sink and drain the trap and start looking through all the junk in the trap, because I had so much time invested in the inclusion.”Once Shirey recovers the inclusion, it’s transferred to a sticky black carbon disk and examined under a scanning electron microscope (SEM) to make sure it’s a sulfide inclusion and not a piece of steel or tungsten carbide from the cracker. Unlike those materials, sulfides look a little “brassy” or yellowish.
He’ll also examine the sulfide for traces of growth morphology and crystallography. Mineral inclusions can tell researchers a lot about the temperature and pressure conditions in which a diamond formed, the rocks and fluids it grew from, and whether it formed at the same time as its diamond host.
Shirey also wants to determine whether the sulfide is from an eclogitic or peridotitic source rock. Peridotitic sulfides contain higher levels of nickel and lower levels of iron. It’s important to make the distinction, Shirey tells us, because that will decide the composition of the special tracer he uses in the “wet” stage of the analysis.
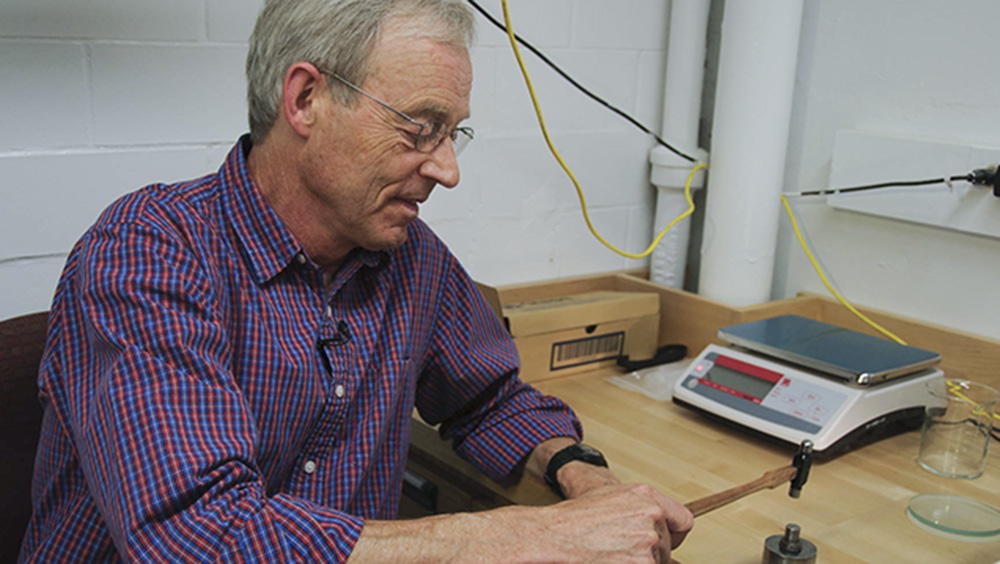
His next step is to wash and rinse the inclusion with ethanol in a tiny beaker, in preparation for weighing it in an extremely sensitive electronic balance. He then carefully picks up the inclusion with a needle and transfers it to a little foil weighing boat that itself weighs a fraction of a milligram.
The weight of the inclusion is especially important, because it controls the exact amount of a special chemical tracer—called a “spike”—that Shirey has to add for the next stage of the work. The spike contains a precisely calibrated mixture of different isotopes of rhenium and osmium.
This is a standard procedure, much used in geochemistry, called isotope dilution. The sulfide inclusion contains tiny amounts of a radioactive isotope of rhenium (187Re), which decays extremely slowly over many billions of years to a stable isotope of osmium (187Os). As the rate of radioactive decay is known, it’s possible to calculate the age of the diamond based on the ratios of 187Re and 187Os present.
Isotope dilution allows researchers to estimate the amount of rhenium and osmium isotopes present—to a very high degree of accuracy—by adding a precisely calibrated solution of different isotopes of each element—the spike—and mixing it very thoroughly with the rhenium and osmium in the inclusion.
Because Shirey knows the weight of the inclusion and the exact amount of spike added, he can calculate the amounts of each rhenium and osmium isotope present from their ratios at the end of the chemistry process without having to recover everything.
Here’s an illustration of the principle behind isotope dilution: Imagine you have a large cardboard box filled with a mixture of white and a smaller number of orange Ping-Pong balls. In this simplified analogy, the cardboard box is the inclusion, the orange balls represent the osmium isotope we want to count, and the white balls stand in for everything else in the inclusion (in other words, the “matrix”).
You can calculate the number of orange balls to the highest degree of accuracy possible without counting every single one. One way is to add a known number of blue Ping-Pong balls—say 100—and mix them thoroughly with all the rest. These blue balls represent the chemical spike.
Once you’re sure all the balls are thoroughly mixed, or diluted with the other colors, take a sample of 50 balls out of the box and count the number of blue ones. From this sample, you can work out the ratio of blue balls to the total number of Ping-Pong balls in the box, as well as the orange ones you need to count. From that information, you can calculate the totals for each color very accurately. Exactly the same principle applies to the isotopes of rhenium and osmium in a diamond inclusion.
After weighing the inclusion, and adding a precisely calibrated amount of the spike, Shirey adds acid to dissolve the inclusion and thoroughly mix its constituents together with the spike.
Separating Rhenium and Osmium
This sequence of videos starts with Dr. Shirey weighing the sulfide inclusion recovered from the diamond. Next, he takes us through the chemistry necessary to separate the radiogenic isotopes of osmium and rhenium from the inclusion for age dating.
Once the acid has dissolved the inclusion, Shirey has to distill out the osmium and rhenium content. With osmium, this is pretty straightforward: “We distill it by vapor transport to a little button of hydrobromic acid, and the rhenium will stay back in a drop of chromic sulfuric.”The rhenium requires a little more work: “We take this chromic sulfuric out, we reduce it, and it will go from brick red to green, and we put it then through an anion exchange column, which is like a Brita filter, but cleaned up and perfect for our work. And what it’ll do is pass everything through the column, retaining the rhenium.”
Shirey then takes the rhenium from the column and dries it down to prepare it for analysis. Now that the osmium and rhenium are separated, he can begin the final part of the process, which is to run both on sophisticated laboratory instruments called mass spectrometers. To avoid any cross contamination, each metal is run on a separate instrument, which counts the different isotopes—or masses—of each element. Shirey tells us, with something akin to relief, “From that we have the isotopic composition, and we can calculate the age…and that’s the end of the work.”
“So we do that on every inclusion,” he adds, “as long as it’s big enough to move around so that we can do the distillation—and from that, believe it or not, we get the age.”
The rhenium-osmium decay system plots as a line on a graph. Each inclusion plots as a data point, on or very close to the line.
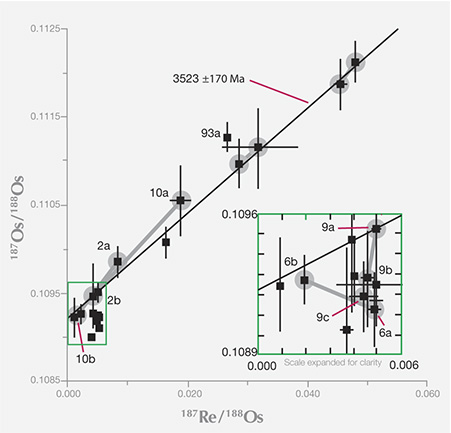
Researchers use the proportion of rhenium and osmium isotopes in each sulfide inclusion
to plot a line called an isochron, which measures the decay of 187Re to187Os. Each data
point represents an age from an inclusion and its diamond host. These diamonds from
Canada’s Ekati mine are the oldest yet known, at 3.523 billion years (3523 Ma on the
graph)—an astonishing age, when the earth was a quarter of its age today. The inset at
right shows detail from the lower end of the isochron.Adapted from Westerlund (2006);
see the Winter 2013 Gems & Gemology, figure 24, p. 211.
to plot a line called an isochron, which measures the decay of 187Re to187Os. Each data
point represents an age from an inclusion and its diamond host. These diamonds from
Canada’s Ekati mine are the oldest yet known, at 3.523 billion years (3523 Ma on the
graph)—an astonishing age, when the earth was a quarter of its age today. The inset at
right shows detail from the lower end of the isochron.Adapted from Westerlund (2006);
see the Winter 2013 Gems & Gemology, figure 24, p. 211.
Using Mass Spectroscopy
In this video, Dr. Shirey explains the use of mass spectrometry to count the isotopes of osmium and rhenium recovered from the sulfide inclusion. This is the final stage of the analysis that began with selecting diamond crystals with suitable sulfide inclusions from working mines. Shirey and his colleagues will use the data captured on the relative amounts of each isotope to determine the age of the inclusion and therefore the diamond itself.
Results from Research
Shirey reckons his biggest contribution to this research area, with his Carnegie colleagues, is development of the rhenium-osmium dating system, which allows dating of individual diamonds. The ages of the diamonds and the chemistry of their inclusions allow researchers to make inferences about the way continents are built and the onset of the earth’s tectonic processes.Shirey’s early work with diamonds focused on one of the world’s best known mining areas, the ancient rocks at the heart of southern Africa, and De Beers’s mining empire: the Kaapvaal craton. The pattern of diamond occurrences and their ages from east to west across the landscape spoke to him about the structure of the deeper continent beneath his feet.
“What really turns me on about it is taking individual diamond measurements and putting them together in a large picture, so that the age distribution would tell us something fundamental on the scale of a continent—or something fundamental on the scale of the earth and a billion-year type of time history. That’s what really gets me excited,” he says.
In the case of the Kaapvaal craton, the diamonds tell Shirey that something changed in the earth’s history about three billion years ago. He and his colleagues propose that this marks the onset of what’s called the Wilson Cycle, the beginning of plate tectonics and subduction of oceanic crust. At the center of the craton lies the graveyard of an ancient ocean that closed when two continental blocks collided—one from the east and one from the west. And the diamonds in the west are different from the ones in the east.
All of the three-billion-year-old diamonds are on the west side of the craton, which agrees with Shirey’s theory about subduction of oceanic crust under the west side. By contrast, there are no diamonds older than three billion years east of the junction—or suture—between these two ancient continental blocks, “and that perfectly explains the type of diamond distribution that you see,” says Shirey.
Shirey explains that diamonds older than three billion years completely lack eclogitic inclusions. This means basalt from subducted oceanic crust had not been thrust deeply enough under the continental keel to be retained and converted into eclogite—a high-pressure metamorphic rock that hosts diamonds. After this date, scientists start to see diamonds with these types of inclusions. And that’s a big important discovery,” says Shirey.
Canadian Diamonds Are Ancient
Another landmark discovery relates to the age of Canadian diamonds. Shirey notes that his group at Carnegie has worked on the country’s two biggest mines, Ekati and Diavik. A Ph.D. student studying Ekati’s diamonds discovered they were the oldest diamonds yet analyzed, at around 3.5 billion years old.The chemistry of these Ekati diamonds indicated an early form of subduction. Shirey and his fellow scientists at Carnegie suggest that the subduction process forming them represents some of the earliest evidence of plate tectonics on the surface of the earth. Shirey mentions that another Ph.D. student working on diamonds from Diavik corroborated their ancient age, but found evidence they were sourced from a deep-seated plume in the earth’s mantle.
“What’s unique about the Canadian occurrences that we’ve looked at so far is their antiquity and the fact that they give us a complicated story,” says Shirey. “We think that there was more than one process to make diamonds in this early Archean period, and that’s actually a very interesting and exciting discovery.”
Superdeep Diamonds from Brazil’s Juina Field
Superdeep diamonds are one of the most exciting finds of the last 15 years, says Shirey. These diamonds carry minerals in them that aren’t found at shallower levels, including high-pressure forms of garnet and olivine that cannot form at shallower depths. The hypothesis is that these diamonds grow from organic material carried down as deep as 700 km into the mantle by subduction. They also have a characteristic irregular internal growth structure that hints at their turbulent origin deep in the earth’s convecting mantle. Superdeep diamonds sample the earth’s mantle at greater depths than scientists have seen before, and they will undoubtedly provide insight about the way the earth recycles carbon. Shirey and a number of his Carnegie colleagues are actively collaborating with researchers at other institutions on this topic. For more details, please see his Carnegie page on superdeep diamonds and mantle convection.
These tiny superdeep diamonds formed at significantly greater depths in the earth’s mantle than most diamonds. Here, they’re mounted for analysis in an electron microprobe. Pedro Padua © GIA, courtesy Carnegie Institution of Washington
Rewards of the Job
Shirey tells us he loves his work because it’s so diverse: “I get to go in the field, to talk to geologists. I get to take specimens and take them apart, to design ways to cut the smallest diamonds.”These challenges stimulate Shirey: “It’s being inventive and crafty, when it comes to manipulating the specimens, but I have to be a chemist as well—I actually had to do the development work on the chemistry. It’s all so different, which is one of the reasons I love doing what I do.”
In the end, he realizes that all this work passes for naught if it’s not communicated in a way that engages the audience. “We have to write the stuff up, communicate our results and tell the story in a way that people find interesting. At the end of the day, if no one wants to hear what you have to say, it doesn’t do any good. So if you don’t make the story interesting, no one’s going to read it.”
Shirey reminds us that research work of this kind is highly collaborative, involving scientists from different disciplines and many partner institutions around the world. He is modest about his contributions. “Most of the work is not done directly by me, but by people that I work with. Almost all the work is done by visitors to the lab, or postdocs that we’re trying to train.
“That’s actually a fun part about my job,” he continues, “I get to be a teacher and a mentor, and that turns out to be a lot of fun.”
About the Authors
About the authors: Duncan Pay is editor-in-chief of Gems & Gemology, and Pedro Padua is a video producer at GIA content development in Carlsbad, California. Dr. Jim Shigley is a distinguished research fellow at GIA’s Laboratory in Carlsbad.
Acknowledgements
The authors would like to thank Dr. Steven Shirey, senior scientist at the Department of Terrestrial Magnetism of the Carnegie Institution of Washington D.C., and all his colleagues there for their help and courtesy during our visit.