Carnegie Scientists Use Diamond Presses to Study Planet Formation
February 28, 2014

For Valerie Hillgren, a research scientist at the Carnegie Institution of Washington’s Geophysical Laboratory, the presses that create these conditions are a means to another end: studying how the terrestrial planets of our solar system might have formed.
We’re standing in a large machine room Geophysical Laboratory with Hillgren and her colleague, Dr. Steven Shirey, from the Carnegie’s Department of Terrestrial Magnetism.
The air rings with the hum of heavy equipment and the rhythmic tick of incremental pressure building in a myriad of pipes. The smell of oil is strong, and dials line the walls. Around us are four enormous multi-anvil presses—each the size of a small Volkswagen—that researchers use to simulate conditions deep inside the earth or other planets. Each apparatus can bring to bear pressures equal to that at the core of the planet Mercury.
The interiors of these machines can accommodate pressures measured in gigapascals (GPa). They’re capable of 25 GPa, the equivalent of 700 km (435 miles) beneath the earth’s surface. Pressures of this magnitude allow scientists to study rock-forming systems in the earth’s mantle.
Although this sounds impressive, it’s only a fraction of the depth of the earth’s layers: the center of the earth’s core is 6,378 km (3,963 miles) below the surface.
“When you want to start looking at things that happen in the deep earth, well, 25 GPa only gets you to the top of the lower mantle,” says Hillgren.
Just for comparison, the pressure inside the earth’s core is 360 GPa or 3.64 million bar. The pressure at the earth’s surface, about 1 bar, equals 0.0001 GPa.
HPHT Research
Valerie Hillgren was able to combine the two subjects she loved, astronomy and geology, into a dream job: studying planetary geology at Carnegie using the Geophysical Lab’s HPHT presses. Pedro Padua © GIA, courtesy Carnegie Institution of Washington.
Hillgren’s work focuses on separation of a planet’s mantle and core, specifically when they start to differentiate into metal-rich and silicate-rich portions. “What we’re looking at is how the chemistry of different elements is partitioned between the mantle and the core of a planet, and how that happens under higher pressure,” she says.Hillgren tells us that the experiments can run anywhere from a few minutes to a day or two, depending on the objectives. “We usually start out with mixtures of oxides and metals in proportions that we think are reasonable for the compositions of the planets,” she explains, “and we put them inside the capsule and heat it up.”
After sufficient heat and pressure, she notes, “It separates into a metal portion and a silicate portion, and then we recover that and study the chemistry.”
In this sequence of photos, you’ll take a glimpse at the inner workings of the Geophysical Lab’s multi-anvil presses.
Hillgren adds, “We look at what our results tell us about the materials that must have formed the planets, what sort of conditions must have existed, when the cores segregated, and perhaps also what has happened after the segregation of the core.”“One of the things we can look at to understand that process is the chemistry of the mantle, which leaves a signature behind from when the metal segregates,” she continues. “So we just try to recreate that by using these presses, here, looking at the conditions in the deep interiors of the planets.”
Currently Hillgren is studying the solar system’s innermost planet: Mercury. “Mercury is a very unusual planet with proportionately a very large metal core compared to other similar terrestrial planets in the solar system—Mars, Earth, and Venus.
“It’s a mystery—why does Mercury have such a large core? Is it the innate chemistry of the planet, or is it some process that happened after the planet formed?”
Hillgren and her colleagues consider the compositions of the various meteorites as potential starting materials for planetary formation: “We have lots of different meteorite classes, and we’re looking to see if any of those make sense to be the starting materials that Mercury was made out of.”

The Chergach meteorite, of which this is a part, fell in Mali in 2007. It’s representative of a class of meteorites called chondrites. These stony (non-metallic) meteorites have not been modified by melting and are considered pretty close to the starting material for many of the planets. Valerie Power © GIA, courtesy H. Obodda Precious Gems and Minerals.
She explains that they’ll experiment with different pressure and temperature conditions. “If we apply different conditions to materials, do we end up with different compositions of mantles and cores? We look at not just the earth, but other planets.”We ask Hillgren which presses she uses. With a laugh she tells us she uses them all. “Depends on what pressure I want to go to! Right here is a piston cylinder, which can go to 2–3 GPa—which would be very shallow. The other presses are multi-anvils, where we can go up to 25 GPa if you want to go deeper.
Hillgren says the interesting thing about Mercury is its core-mantle boundary. “So if you want to look at the segregation of the core,” she explains, “it’s only 7 GPa, which makes it a very easy and accessible planet to study using these presses with very routine methods.”
Finally, we ask Hillgren how she became interested in this field. She feels very fortunate things turned out the way they did: “When I was young, I was always interested in astronomy, and once I got into college, I became very interested in geology…In my last year in undergraduate school, they had a course on planetary geology, and for me it was just combining two things that I really loved…outer space and geology.”
To see more on high-pressure research at the Carnegie Institution of Washington’s Geophysical Lab, visit: https://www.gl.ciw.edu/research/high_pressure_research
Other High-Pressure Research at Carnegie
According to Dr. Steven Shirey, the Carnegie Institution’s Geophysical Lab has long been a leader in high-pressure research. It started with experiments in the early 1900s at much shallower pressure and higher temperature to look at rock-forming mineral systems in the earth. Scientists were looking to gain a basic understanding of their thermodynamics to work out how rocks and magmas form in the deep earth.Shirey tells us there hasn’t been a lot of work on natural diamonds at the Geophysical Lab, but that there’s likely to be more in the future. Much of the lab’s work has been focused on synthetic diamonds—especially CVD-grown—and experiments into exotic new materials for use as semiconductors and other technological applications.
Early Research Using the Piston Cylinder
Research in the 1960s used the piston cylinder, a simpler type of high-pressure device. As Shirey describes it, “You have a cylinder, that’s the confining jacket, and there’s an inner ring and then a subsidiary ring and then another ring. These confinement rings are assembled at pressure, so that they can handle the potential expansion as you push the piston.”
Piston Cylinder
Dr. Steven Shirey explains the Geophysical Lab’s early forays into the realm of high-pressure, high-temperature research, beginning with the earth’s rock-forming systems.
“The sample goes in a cylinder of various materials with a central region,” he says. “It’s usually some sort of capsule made out of a noble metal—could be gold or platinum, or it could be graphite. And then there’s a bunch of compressible components—could be salt or pyrophyllite—and then a jacket around that.”Pyrophyllite is a phyllosilicate mineral composed of aluminum silicate hydroxide—Al2Si4O10(OH2)—that’s widely used in high-pressure experiments, both as a gasket material and as a pressure-transmitting medium.
Shirey explains that a resistance heating system goes in through the holes in the cylinder: “When you compress the whole thing, you get the pressure from compression and you get the heating from the application of a voltage across this resistance.”
These devices can get into the 50 kilobar (5 GPa) range or thereabouts, which would be about 150 km (93 miles) inside the earth, he says: “That’s about the top end of a piston cylinder.”
That’s miniscule compared to a multi-anvil press, Shirey says, gesturing at the larger apparatus behind him: “Nonetheless, the early problems attacked with the piston cylinder were basic things—the melting relations of the mantle, the kinds of melts that were formed, and this would simulate a lot of the experimental work you’d see in mantle xenoliths that are brought up in kimberlites.”
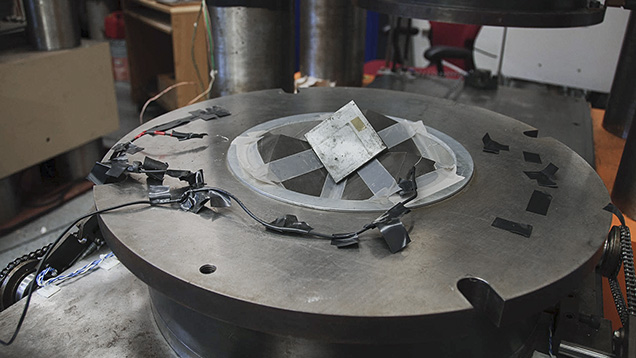
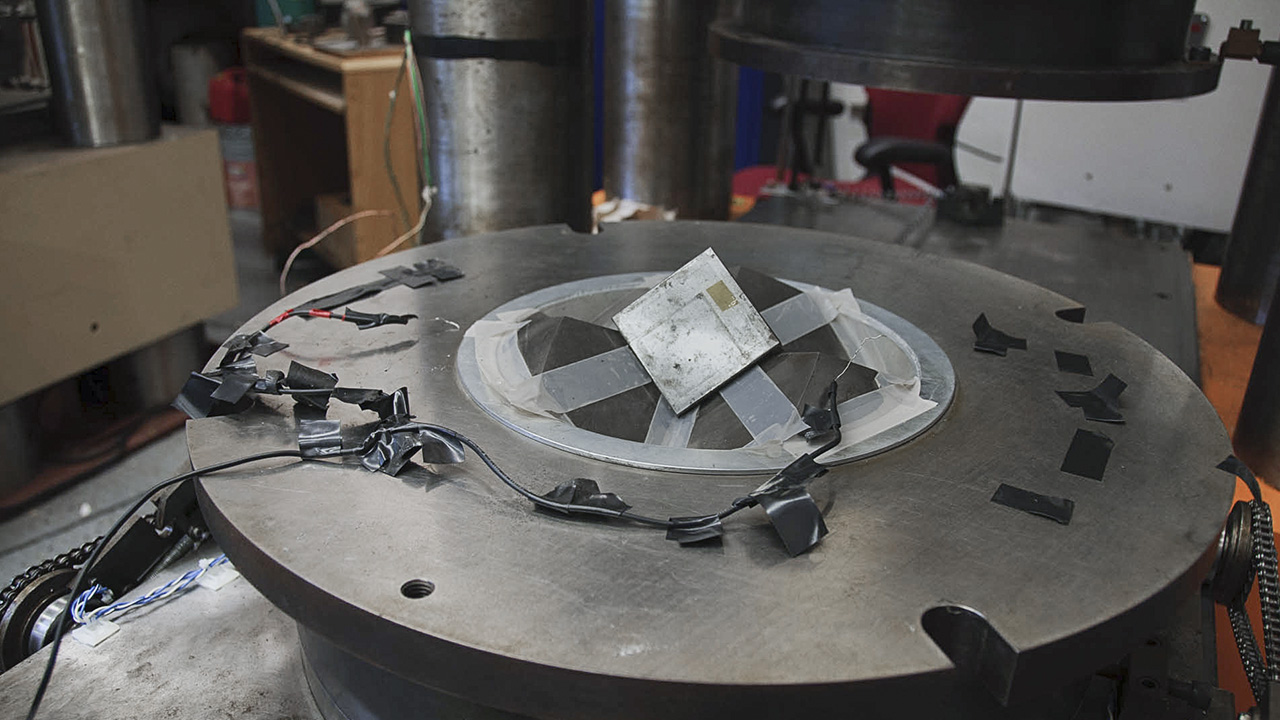
Multi-Anvil Presses
Any work done on natural diamond formation would require use of the multi-anvil presses, which can hold larger volumes of experimental materials, Shirey says. Just like the piston cylinder, scientists can apply a voltage across a resistance to heat the ample to high temperatures to replicate those inside the earth or another planet.
Multi-Anvil Presses
In this video, Dr. Shirey shows us the Carnegie lab’s multi-anvil presses and explains their advantages over earlier devices like the piston cylinder.
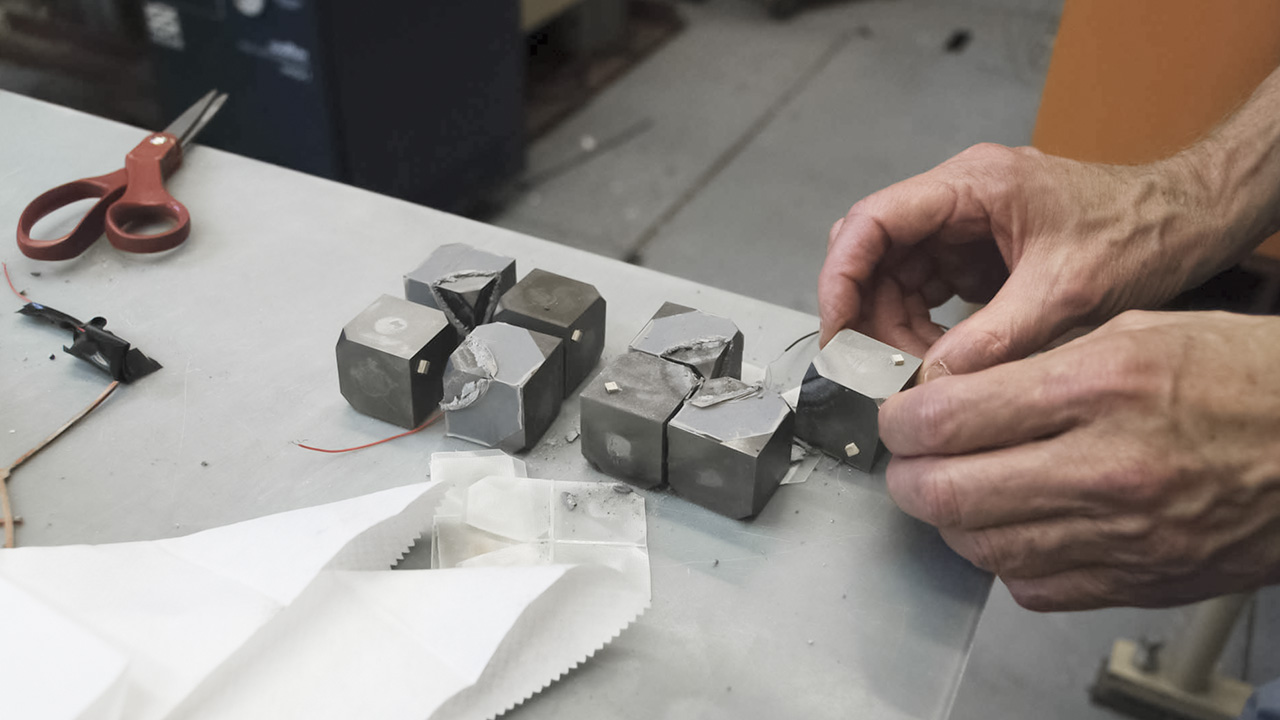
But multi-anvil presses work in a different way, he says: “Here we have these opposed, cubic pieces that float in a larger containment, and then inside them are boron nitride cubes that then have a cutout in the corner and the sample goes in the corner of the cubes.”Shirey holds the octahedral sample holder—made from a compound of magnesium and chromium oxides (MgO and Cr2O3)—between his thumb and forefinger. It nests at the center of the eight cubes that might be composed of either boron nitride or tungsten carbide. It’s rather tiny, given the bulk of the press. A diagonal hole pierces the sample holder, and that’s where the actual experiment occurs. Shirey tells, without a hint of irony, that this is a “larger-volume apparatus.”
“The virtue of these guys is that they can go to much higher pressures with a volume that’s big enough to put into an electron microprobe to do a pretty good analysis.”
About the Authors
About the authors: Duncan Pay (dpay@gia.edu) is editor-in-chief of Gems & Gemology, and Pedro Padua is a video producer at GIA content development in Carlsbad, California. Dr. Jim Shigley is a distinguished research fellow at GIA’s Laboratory in Carlsbad.
Acknowledgments
The authors would like to thank Valerie Hillgren, a research scientist at the Geophysical Laboratory, and Dr. Steven Shirey, senior scientist at the Department of Terrestrial Magnetism, both of the Carnegie Institution of Washington D.C., and all their colleagues for their help and courtesy during our visit.