Carbon Isotope Studies Reveal Diamond Growth History
March 26, 2014

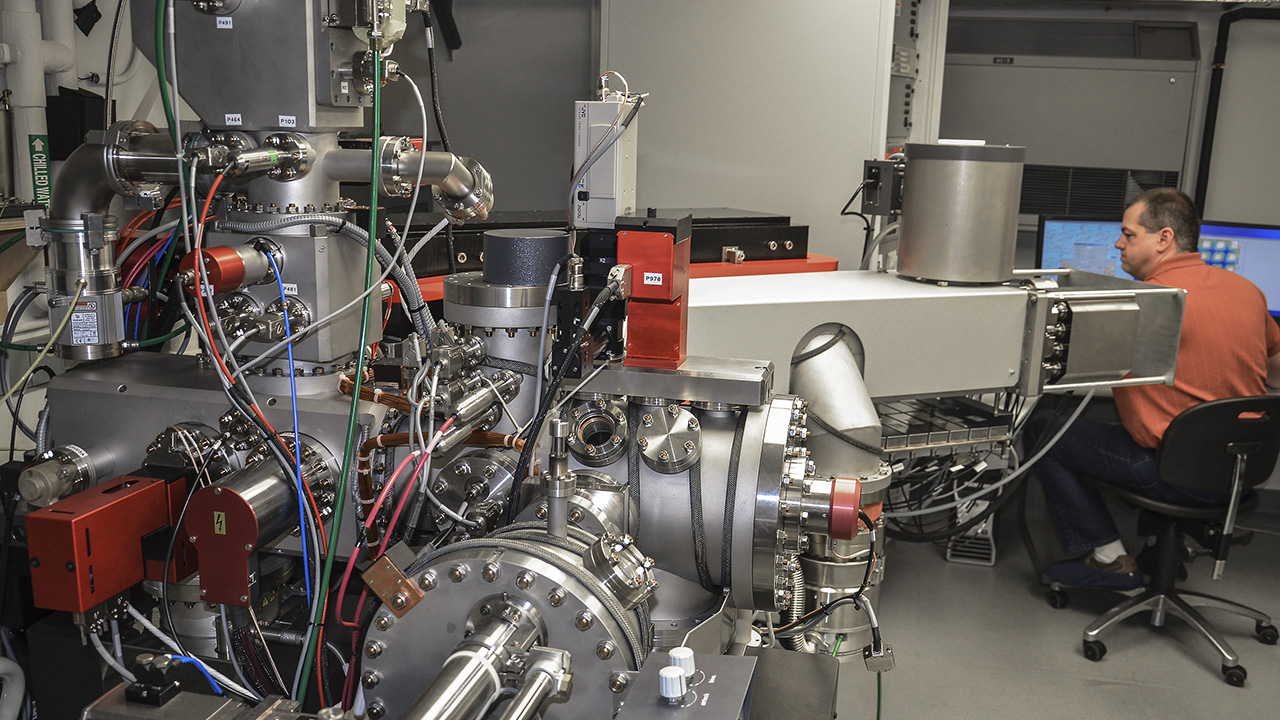
Today, we are in the DTM’s ion microprobe lab. Dr. Wang introduces us to the complex machine humming behind him. Called a secondary ion mass spectrometer (SIMS), it occupies most of the large room we’re standing in.
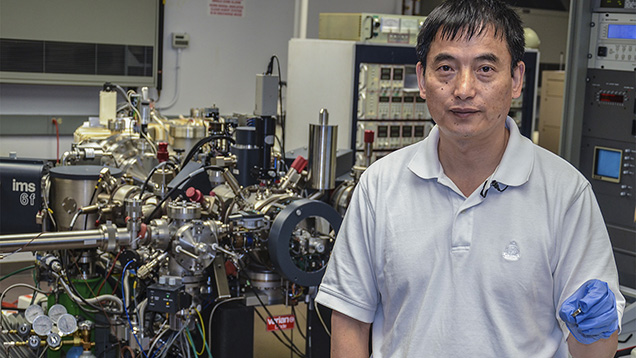
Dr. Jianhua Wang explains operation of the Department of Terrestrial Magnetism’s sophisticated ion microprobe and its relevance to natural diamond research. He has a sample holder in his hand ready to place into the instrument’s vacuum chamber. Photo by Duncan Pay/GIA, courtesy of Carnegie Institution of Washington.
The Ion Microprobe Explained
Dr. Wang outlines the “business end” of the ion probe: a compact mass of complex cylinders and pipes that resembles a partially disassembled jet turbine. This area contains the ion gun that bombards the sample and the vacuum chamber that holds it. Beyond this is a long “flight tube” that curves around the walls of the room, through an immense magnet the size of a standard refrigerator, to a series of detectors at the end. It’s a very impressive-looking instrument.
Ion Microprobe
Join Dr. Jianhua Wang as he introduces us to the Department of Terrestrial Magnetism’s ion microprobe.
The Ion Microprobe Explained
Dr. Wang gives us a quick outline of the ion microprobe. He tells us the instrument has a primary ion "gun," which generates an ion beam. An ion is an atom with a positive or negative electrical charge. In this case, it's a positive ion of the rare metal cesium (133Cs).The primary ion beam passes through a set of electromagnetic lenses, he explains. They focus it to a diameter of 15 micrometers (0.0015 cm), and accelerate it toward the sample in the instrument’s high-vacuum chamber. The impact of the cesium ion beam causes the sample to emit a stream of its own ions in response.
Another set of electromagnetic lenses focus the ions emitted by the sample into a secondary ion beam. It’s this feature that gives the SIMS instrument the first part of its name.
Once the beam has been directed, it’s fed into a mass spectrometer to identify the ions produced by the sample. The mass spectrometer separates the secondary ions by their charge and mass into the different isotopes. For diamond, Dr. Wang typically measures carbon or nitrogen, the predominant trace element in diamond.
The mass spectrometer operates on the principle that two ions with the same mass and charge move in the same path in a vacuum when subjected to the same electromagnetic field. Ions with different mass-to-charge ratios are deflected to some degree. The mass spectrometer separates the ions into different masses and counts them in an array of detectors. A computer screen displays the results.
With this machine, Dr. Wang explains, it’s necessary to change the magnetic field to detect isotopes of different masses: “If you want to measure carbon isotopes and nitrogen isotopes using this machine, you have to do it in two different sessions, because nitrogen is a trace element in diamond and carbon is the major element.”
“So you have a lot of carbon signal,” he says. “You do carbon isotopes in the one session, and you measure nitrogen isotopes and nitrogen concentration in a different session.”
Carbon Isotope Research Illuminates Diamond’s Geologic History
Of all Dr. Wang’s research, the topics of most particular interest to us are diamond’s place in the earth’s carbon cycle, and the origin of the carbon that makes up the fabric of a diamond crystal.Dr. Wang’s work is complementary to Dr. Shirey’s. While Dr. Shirey focuses on age dating of tiny sulfide inclusions, Dr. Wang works on isotope studies of the carbon and nitrogen in the polished diamond plates that Dr. Shirey produces before cleaving them to recover the inclusions.
Over the last decade, scientists like Dr. Wang have made major advances in understanding the sources of carbon in diamonds. Carbon occurs as two stable isotopes, 12C and 13C. (An isotope of a chemical element is simply an atom with the same atomic number as that element, but with a slightly different mass, resulting from a different number of neutrons in its nucleus.) At the earth’s surface, 12C is almost a hundred times more abundant (98.9% compared to 1.1%).
There are really only two sources for the carbon in natural diamond. It can either come from carbon that has never left the earth’s mantle, which scientists call “primordial,” or it can be “recycled” carbon that has been released from the mantle and incorporated into organic matter—like bacteria or algae—or subsequently formed carbon-bearing minerals like graphite, or carbon-rich rocks like limestone or marble. These materials have then been carried back into the earth’s mantle by subduction, releasing the carbon for diamond formation.
Broadly speaking, scientists classify natural diamonds into two broad categories depending on their geologic origin. Those that source their carbon from the mantle are peridotitic (P-type), and those that contain subduction-recycled carbon are eclogitic (E-type).
Carbon that has been recycled at the earth’s surface tends to have a much higher proportion of 12C to 13C. That’s because the biochemical reactions that fuel living systems generally favor the lighter isotope of carbon. Depending on whether the diamond originated from mantle carbon or recycled carbon, the ratios might be very different, reflecting its geologic history. Dr. Wang’s ion probe can count the different carbon isotopes in diamond, allowing him to determine the ratio of 13C to 12C.
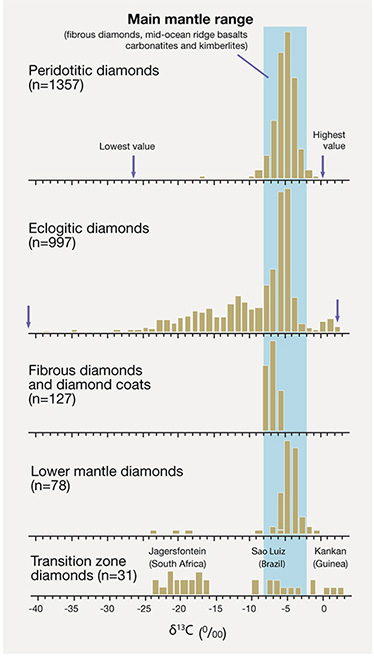
Carbon isotope composition tells us a lot about a diamond’s geologic history. This illustration shows the difference between some of the main diamond types. δ13C is the 13C/12C ratio measured against a reference standard and deviating from this standard by 0.1%. Note the negative scale and how eclogitic diamonds extend to much lower δ13C values than peridotitic diamonds; n = the number of analyses. Diamonds sourced from mantle carbon (P-type, top graph) have a relatively restricted range of values. Those sourced from subducted recycled carbon (E-type, second graph from top) tend to have a much wider range, though both overlap. Adapted from Cartigny (2005); see the Winter 2013 Gems & Gemology, figure 21, p. 206
Peridotitic diamonds show a narrow range in carbon isotopic composition (given in the delta notation, δ13C, where 13C/12C is referenced to a standard and expressed in ‰) of –10 to –2 δ13C, with more than 95% falling in the main mantle range of –8 to –2.Eclogitic diamonds show a much wider δ13C range (from –42 to +3), even though many also overlap the main mantle range.
Carbon in Diamond
In this video, Dr. Wang explains the significance of his carbon isotope work and how the results inform our understanding of natural diamond’s origin.
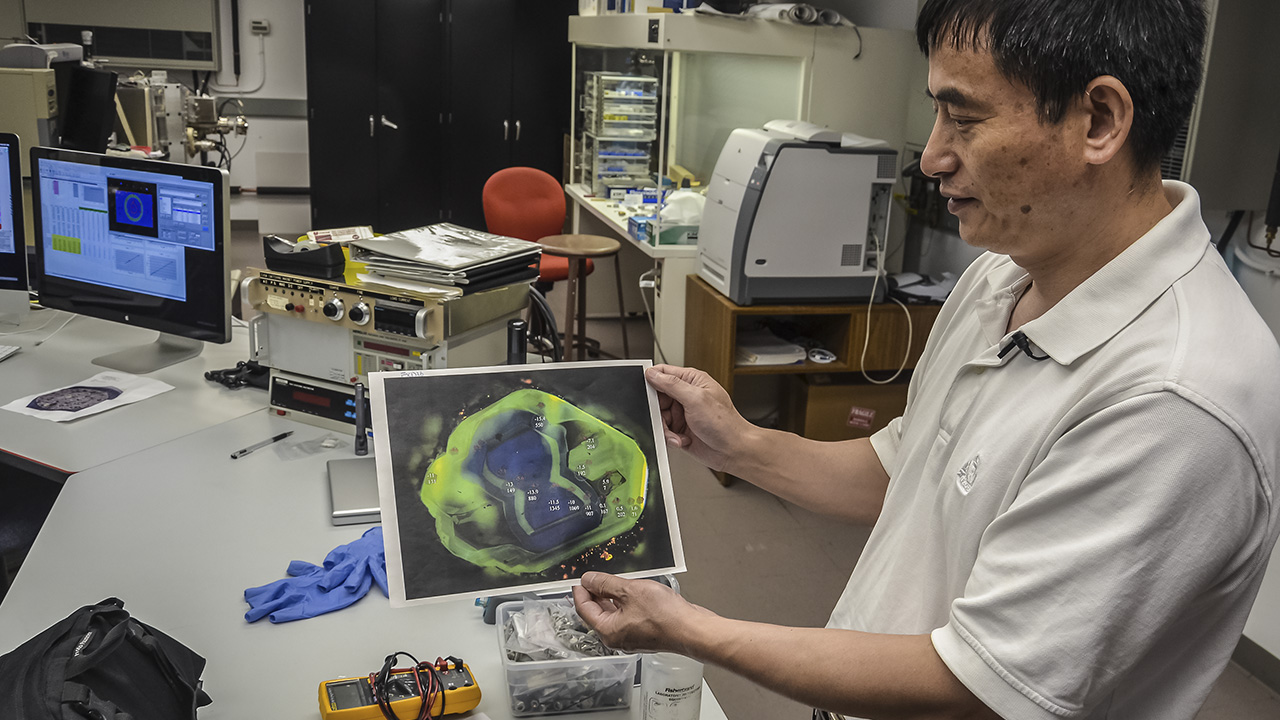
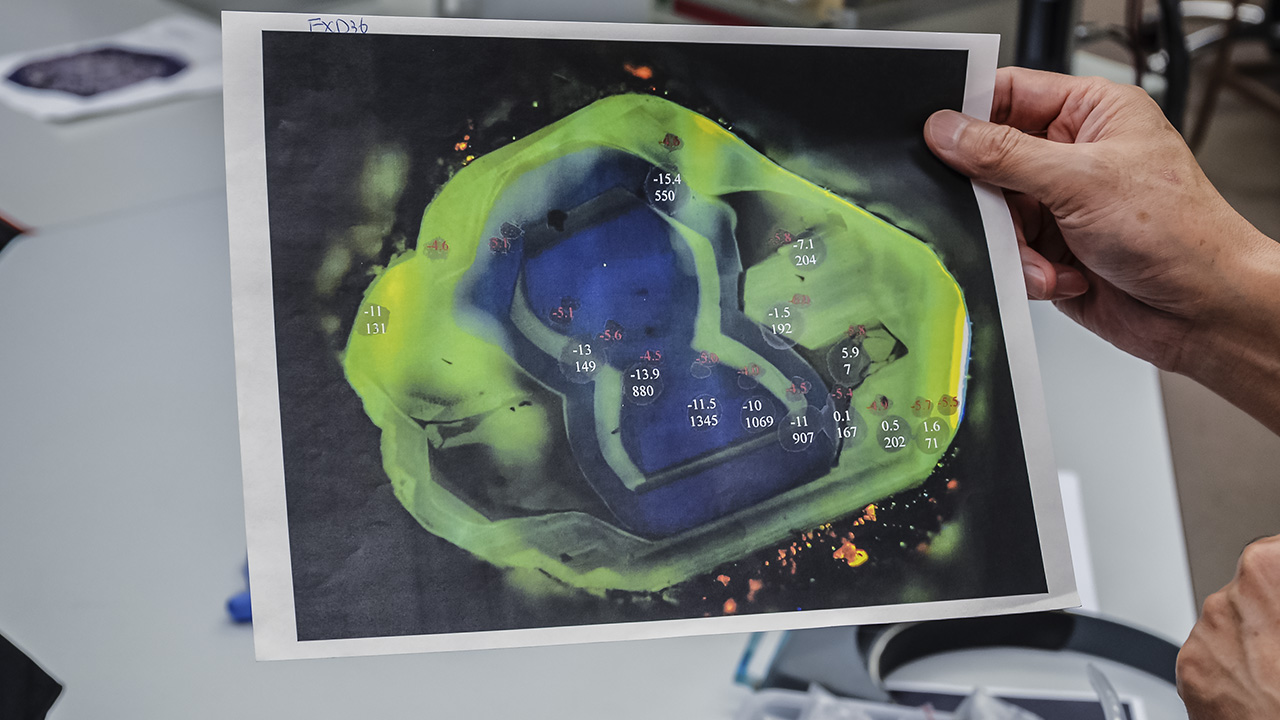
So what can studying the carbon isotopes in diamond tell us? “It’s the deepest aspect of how Earth’s subduction works,” Dr. Wang replies.He shows us a printout of a cathodoluminescence (CL) image of a diamond plate cut from a crystal with a complex growth history. The printout contains an enlargement of a diamond plate just a few millimeters across.
He tells us that the different colors represent the different concentrations of nitrogen throughout the plate, which are normally invisible without CL. “You can see a multi-growth pattern of the diamond, with variations in the amount of nitrogen, and we can use the ion probe to measure carbon isotopes in different regions.”
Each point sampled by the ion probe appears as a tiny spot 15 micrometers (0.0015 cm) across. Figures next to each spot note the isotope concentrations. Dr. Wang points to the trail of sample points and says, “From this information, we see variation of the carbon isotopes.
“If it changes a lot, we can infer there have been different carbon sources in the fluid from which the diamonds grew. In this case, all the carbon is from the mantle—it’s very close to the primordial composition of mantle carbon isotopes.”
On the other hand, Dr. Wang informs us, “If you see very negative carbon isotope composition—say, -24 δ13C—in most cases, this is carbon of organic origin, so they might be coming from the surface of the earth. This could indicate the subduction of oceanic crust from the surface deep into the mantle.
“This technique can show you where the carbon in a diamond came from,” he continues. “Whether it’s from the top—the surface of the earth—or whether it’s of deep mantle origin.”
This sequence of images introduces you to one of the key analytical instruments used for diamond-related research at the Carnegie Institution’s Department of Terrestrial Magnetism.
Superdeep Diamonds from Under Brazil
Dr. Wang refers to his recent research paper from 2012, with the diamond research group from the University of Bristol (UK), where he and his colleagues analyzed some unusual diamonds from Brazil. “We found out some diamonds had a very strong signature of organic origin and that some of the inclusions in them were formed very deep in the mantle, like 800 kilometers deep.“From these inclusions, we can infer that the (carbon) source is from the earth’s surface, but that the diamonds formed deep in the mantle. So that tells us that the subduction went all the way down to the deep mantle.
“The diamond tells us the history of the carbon coming from the surface, how it went down to the mantle and formed the diamond, and the kimberlite brought them to the surface and we got the diamonds and measured them in this lab.”
Like so much of research, it’s conducted with colleagues, especially in this case with Drs. Shirey and Hauri: “Steve has been measuring the sulfide composition in the diamond to find out when the inclusions in diamonds were formed, and Erik has been measuring water content to see how much water is in the deep mantle,” he says. “It’s a collaboration with other people to get this research done.”
Capabilities of Carnegie’s Ion Microprobe
Dr. Wang explains that this type of instrument is mostly used by the semiconductor industry to measure the implant of different trace elements into silicon wafers.
Unique Instrument
Dr. Wang outlines some of the capabilities of the Department of Terrestrial Magnetism’s ion microprobe and explains why it would be very costly to replace.
“In geological and cosmological science,” he tells us, “we’re using it to do trace element analysis in minerals and rocks, including measurement of water in Martian meteorites, and in moon rocks.” Additional uses haved used DTM cosmochemists to look at particles actively collected from the tail of comets, interplanetary dust particles that constantly rain down on Earth from outer space, meteorite residues that contain refractory grains made in distant stars outside our solar system, and organic components in meteorites blasted from Mars.To keep a complex and versatile instrument running at state-of-the-art precision requires considerable institutional resources and a team of users. Besides Dr. Wang and his Carnegie colleagues Drs. Alexander, Hauri, Nittler, and Shirey, there is a bevy of post-doctoral researchers and visiting scientists from around the world who routinely come through the ion microprobe facility. It is an exciting place, indeed.
We can’t resist asking the value of this unique instrument: “When we purchased this instrument, it was about $1.5 million,” Dr. Wang says, gesturing at the French-built core of the machine with its ion gun and sample chamber.
With a sweep of his arm he takes in the rest of the ion probe, with its long flight tube and immense magnet. “This is an in-house development—we spent probably a few hundred thousand dollars, but if you buy a commercial, same-size version of the instrument, it would be like $4 to $4.5 million!”
Next-Generation Microprobe
In the next room, Dr. Wang gives us a glimpse of the next generation of microprobe, the nano-SIMS. It has the benefit of a much smaller beam size and a much larger magnet. The magnet allows it to have an array of seven detectors, so it’s possible to measure seven different masses (isotopes) simultaneously. The larger SIMS machine has only one detector, so if you want to measure different isotopes, you have to measure them in separate sessions by switching the magnet to accommodate the mass of each isotope.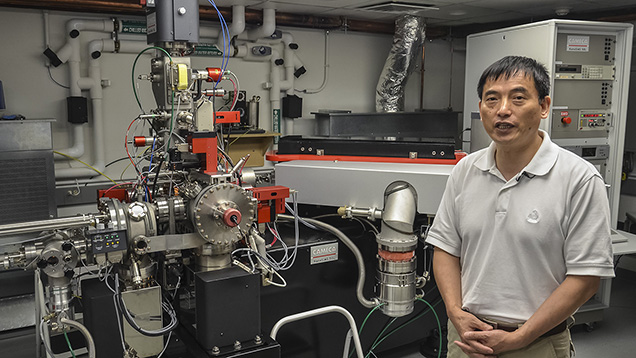
Dr. Wang explains the operation of the lab’s newer ion microprobe: the nano-SIMS, which is capable of analyzing even smaller samples, including tiny diamonds in meteorites and minute inclusions in gem diamonds. Photo by Duncan Pay/GIA, courtesy of Carnegie Institution of Washington.
The newer instrument’s vacuum chamber is also superior, which is important for measuring light elements like hydrogen. Its two-stage sample chamber allows researchers to achieve higher vacuums and exclude almost all contaminants. The small beam size permits analysis of the tiniest grains in meteorites so researchers can sample the supernova remnants captured in these pre-solar system bodies.Besides his interest in diamond isotope research, one of Dr. Wang’s other open questions, asked with his DTM colleague Dr. Erik Hauri, centers around water on the moon. Analysis of moon rocks and volcanic glasses in moon rock minerals has shown higher water content than anticipated. Current theories suggest that a Mars-sized planetary body collided with ancient Earth and formed the moon as a result, and that somehow the moon was able to retain water locked up in its rocks and minerals.
Once again, we’re reminded that analysis of nature’s smallest features—tiny inclusions in diamonds, or traces of dust from the early solar system—can lead to groundbreaking discoveries on the scale of our planet’s history, or even larger. We’ll aim to keep in touch with Dr. Wang’s research and update you in the near future.
About the Authors
About the authors: Duncan Pay is editor-in-chief of Gems & Gemology, and Pedro Padua is a video producer at GIA content development in Carlsbad, California. Dr. Jim Shigley is a distinguished research fellow at GIA’s Laboratory in Carlsbad.
Acknowledgments
The authors would like to thank Dr. Jianhua Wang, senior scientist at the Department of Terrestrial Magnetism of the Carnegie Institution of Washington D.C., and all his colleagues for their help and courtesy during our visit.