Fluorescence Characteristics of Two Copper-Diffused Plagioclase Feldspars: Labradorite and Andesine

ABSTRACT
Natural-color labradorite sunstone from Oregon has been available for more than a century. In the first decade of the 2000s, a type of “red andesine feldspar” was unveiled at the Tucson gem shows, and its origin remains an open question. There is still no convenient method that can quickly and reliably determine whether a red andesine feldspar has been treated. For this study, both colorless labradorite and light yellow andesine feldspars were copper diffusion treated to modify their color to mimic natural labradorite sunstone. Systematic studies of the fluorescence characteristics of both untreated and treated labradorite and andesine feldspars as well as natural gem-quality sunstone from Oregon and Ethiopia were conducted. Under 320 nm excitation, strong fluorescence emission near 394 nm and weak fluorescence emission near 554 nm were observed in the spectra of copper-diffused red labradorite and andesine feldspar. For comparison, natural red and green labradorite sunstone material from Oregon and Ethiopia were tested, and these exhibited substantially weaker fluorescence emission around 394 nm, similar to the untreated nearly colorless labradorite and light yellow andesine feldspar samples. This fluorescence characteristic, due to the presence of Cu+ ions in the plagioclase crystal structure, can potentially help to identify copper diffusion treatment.
Feldspar, with orthoclase (KAlSi3O8), albite (NaAlSi3O8), and anorthite (CaAl2Si2O8) as end members, is one of the most important rock-forming minerals in the earth’s crust, and those of high quality can be used as gemstones. The main feldspar gems on the market include moonstone, sunstone, amazonite, and iridescent rainbow labradorite. Among them, sunstone exhibits a strong golden and red metallic luster due to microscopic metallic inclusions. But there is a special kind of labradorite sunstone—Ab50–30An50–70, where Ab is albite and An is anorthite—that not only shows “schiller” (aventurescence) but also presents brilliant red and green bodycolors. Currently, the reported localities of this kind of natural labradorite sunstone are the U.S. state of Oregon and the Afar region of Ethiopia (Johnston et al., 1991; Kiefert et al., 2019; Sun et al., 2020). This material is highly valued and favored by many gem carvers and collectors for its unique optical properties.
Research on the color origin of labradorite sunstone has been ongoing since its discovery. In the beginning, researchers identified copper as the color-causing element in red labradorite sunstone. Quantitative analysis of labradorite sunstone with different colors verified this, but classical crystal field theory (absorption bands of Cu2+ or Cu+ ions) cannot provide a reasonable explanation. Previous studies compared the absorption spectra of red labradorite sunstone with that of “ruby glass” containing copper nanoparticles and found that the absorption peaks of these two different materials are very similar (Hofmeister and Rossman, 1985; Rossman, 2011). This result suggests that the presence of copper nanoparticles contributes to the color in labradorite sunstone. Nishida and Kimata (2002) analyzed copper microinclusions in natural labradorite sunstone by combining chemical composition results with characteristic X-ray imaging by element. They summarized that the transparent red color of the Oregon sunstone is due to the distribution of mixtures of both native copper and cuprites in oval-shaped thin films, while the transparent green color is due to the distribution of similar mixtures in short microcolumns. However, due to technical limitations, the existence of copper nanoparticles in natural sunstone had not been directly verified. In Wang et al. (2019), authors CW and AHS from the present study directly observed the microscopic morphology of copper nanoparticles in natural sunstone by means of focused ion beam and transmission electron microscopy (FIB-TEM).
Because of the high price and market potential of natural sunstone, a new variety of red and green andesine feldspar (Ab70–50An30–50) was unveiled during the 2006 Tucson gem shows (Rossman, 2011). The gem sellers claimed that this red andesine feldspar came from a new locality in China, which attracted the attention of major gem research institutions around the world. Since then, the mining of natural red and green andesine in Tibet has been a subject of controversy (Hughes, 2011a; Schorr et al., 2012). The similar appearances of the red andesine feldspar and natural Oregon labradorite sunstone have led many gemologists to examine whether the Tibetan red andesine feldspar material is natural and untreated using two approaches: analytical technology (Rossman, 2011; Peretti et al., 2011a,b) and on-site inspection (Abduriyim et al., 2008, 2009a,b; Abduriyim and Laurs, 2010; Hughes, 2010, 2011b; Wang et al., 2011). The identification of copper diffusion treatment might be possible using a combination of several advanced techniques, such as elemental fingerprints and isotope ratio determination of copper and argon, but most of these are expensive, destructive, and not widely available.
The treatment methods used in the marketplace to modify colorless plagioclase feldspar to red and green color are not completely understood. Many researchers have tried to generate red and green colors using copper diffusion experiments, typically by placing colorless or light yellow feldspar in a zirconia powder containing a small amount of copper and then performing a high-temperature diffusion treatment (Emmett and Douthit, 2009; Thirangoon, 2009; Wang, 2012; Cao, 2013). By adjusting the diffusion temperature, different shades of red colors can be obtained. Specifically, Emmett and Douthit (2009) treated nearly colorless labradorite feldspar at 1000°, 1100°, and 1170°C for 160 hours. Cao (2013) treated light yellow andesine feldspar at 1100°, 1150°, and 1200°C for 24, 48, and 72 hours, respectively. Except for some microscopic characteristics caused by heat treatment such as black particles, lath-like hollow channels, and pipe-like growth tubes, no direct identification evidence of treatment was reported in these diffusion experiments.
Nevertheless, we still believe that copper diffusion experiments offer the best means of revealing whether red andesine feldspar has been treated by copper diffusion. This research systematically reports on copper diffusion experiments and the fluorescence characteristics excited by the 305–335 nm ultraviolet light, which is seldom used in gem identification.
MATERIALS AND METHODS
Samples. A total of 29 natural nearly colorless or light yellow labradorite and andesine feldspar samples and red labradorite sunstones were used in this study. The near-colorless and light yellow stones were used for copper diffusion experiments, and all samples were analyzed for composition and studied spectroscopically. These samples (figure 1 and table 1) were categorized into four groups, A through D. Group A contained eight nearly colorless labradorite feldspar samples from Oregon, all purchased by one of the authors (AHS) from an Oregon miner at the Tucson Gem and Mineral Show (TGMS). Group B contained four light yellow andesine feldspar samples from Inner Mongolia, China, purchased by one of the authors (QZ) from a miner there. Group C consisted of 13 natural red or green labradorite sunstone samples from different mines in Oregon, on loan from GIA: five from Ponderosa, five from Dust Devil, and three from Sunstone Butte. Group D comprised four natural red labradorite sunstone samples from Ethiopia, on loan from Gübelin Gem Lab. Samples in groups A and B were copper diffusion treated in our laboratory, and the fluorescence and composition characteristics of the samples in groups C and D were compared to the samples in groups A and B.

Copper Diffusion Treatment. The nearly colorless and light yellow labradorite and andesine feldspar samples were precleaned with aqua regia, buried in a diffusant, and placed in a tube furnace for heating in an air atmosphere. In the corundum crucible, the diffusant used was a mixture of CuO powder (0.05 g) and ZrO2 powder (5.00 g), with both powders having a purity of 99.9%. The heating and cooling rates were set to 5°C/min, with a soak temperature of 1170°C and a soak time of 72 hours. After the tube furnace was cooled to room temperature, the diffusion-treated feldspar samples were removed, and all samples were repolished to remove damage and surface contamination.
Characterization Techniques. Images of all the samples were captured in a light box (D55 light source) under identical conditions to compare color changes. Fluorescence spectra were collected using a Jasco FP-8500 spectrofluorometer with a xenon lamp light source. 3D fluorescence spectral data was collected with a 2000 nm/min scan speed. The excitation wavelengths varied from 200 to 500 nm, with a step size of 5 nm and an excitation bandwidth of 5 nm. The emission spectra were collected with a starting wavelength 10 nm longer than the excitation wavelength and up to 750 nm, with the bandwidth set to 2.5 nm and a data interval of 1 nm. The photomultiplier tube (PMT) voltage was fixed at 600 V for all samples to compare the fluorescence intensity. The 2D emission spectra (340–750 nm) were measured with an excitation wavelength of 320 nm at a response time of 0.5 seconds, a scan speed of 1000 nm/min, and a starting wavelength 20 nm longer than the excitation wavelength. For all samples, the parameter settings of the instrument remained unchanged. Rhodamine B was used as a calibrator of fluorescence intensity and dissolved in ethanol to form a solution of 5 μg/mL. Under the same fluorescence test conditions, the emission wavelength of the Rhodamine B solution was 565 nm and the fluorescence emission intensity was 4500 cps.
Trace element analyses were conducted by laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS). Detailed operating conditions for the laser ablation system and the ICP-MS instrument and data reduction were the same as those described by Liu et al. (2008). Laser sampling was performed using a GeoLas 2005 system (laser wavelength of 193 nm, energy density of 14 J/cm2, helium carrier gas, ablation spot size of 44 μm, repetition rate of 8 Hz, and laser pulse of 480). An Agilent 7500a ICP-MS instrument (radio-frequency power of 1350 W and dwell time of 6 ms) was used to acquire each individual data set. Element concentrations were calibrated against multiple reference glasses (BCR-2G, BIR-1G, and BHVO-2G) without applying internal standardization. The values of element concentrations used for the U.S. Geological Survey reference glasses are from the GeoReM database (http://georem.mpch-mainz.gwdg.de). Offline selection and integration of background and analyte signals as well as time-drift corrections and quantitative calibrations were performed by ICPMSDataCal. Two spots were measured for each sample, and the spots were selected in the colored area for the colored samples.
RESULTS AND DISCUSSION
Appearance, Fluorescence Spectra, and Copper Concentration of Nearly Colorless Labradorite Feldspar. According to the LA-ICP-MS data in table 2, the untreated feldspar crystals from Oregon are classified as labradorite, and the average mass concentration of copper is 0.1–18.6 ppmw. The labradorite feldspar crystals were transparent and nearly colorless (figure 2 inset) before the high-temperature copper diffusion treatment. The Oregon labradorite feldspar rough samples used ranged in weight from 1.73 to 4.15 ct. Figure 2 shows the fluorescence spectra of the untreated labradorite feldspar samples, which exhibit weak fluorescence emission peaks centered in the wavelength range from 400 to 420 nm under 320 nm excitation. The intensity of the fluorescence emission peak is 100–600 cps. It must be mentioned that there is another set of fluorescence emission peaks at ~350 nm. These weak fluorescence emissions may be related to the presence of copper or other trace elements contained in the feldspar crystals, but this has not been verified.



Changes in Labradorite Feldspar Appearance, Fluorescence Spectra, and Copper Concentration After Copper Diffusion Treatment. Figure 3 shows the eight labradorite feldspar samples after copper diffusion treatment. To better show the color changes, the surfaces of all samples were repolished after treatment. It can be clearly seen from the figure that a wide variety of phenomena were produced, consistent with those reported by others (Emmett and Douthit, 2009). In some cases, it appears the copper diffuses by bulk (or lattice) diffusion and the red color distribution is uniform; in others, pipe or short circuit diffusion is predominant and the red areas in the samples are intermittently distributed. In addition, the areas that were in contact with the diffusant and have not been repolished show obvious damage and darkening.

From the 3D fluorescence pattern of a red labradorite feldspar diffused in the authors’ laboratory (figure 4), we found that the fluorescence emission consisted of two emission peaks at 389 and 554 nm, with their corresponding optimal excitation wavelengths at 305 and 335 nm. After identifying the main emission peaks, a specific excitation wavelength of 320 nm was chosen to balance the intensity of these two emission peaks. We chose the excitation wavelength of 320 nm so that the fluorescence emissions at both 389 nm and 554 nm could be excited well.


The fluorescence spectra in figure 5 show that all the diffused red labradorite samples exhibit two typical fluorescence emission peaks under the 320 nm excitation. Instead, the fluorescence emissions at ~350 nm in untreated labradorite samples disappeared. The wavelength of the first emission peak is 390.7–396.3 nm with an intensity of 3550–4992 cps, while the wavelength of the second emission peak is ~554 nm with an intensity of 232–620 cps. The spectral parameters are summarized in table 3. The different fluorescence characteristics of the labradorite feldspar samples under 320 nm UV light before and after copper diffusion treatment are shown in the figure 5 inset. The untreated sample shows no obvious fluorescence, but the treated one exhibits intense purple-red fluorescence. Based on the fluorescence spectra or fluorescence characteristics, it is potentially possible to determine whether a labradorite feldspar has undergone copper diffusion treatment. Although we are not aware of any copper-diffused red labradorite feldspar currently on the market, it is indeed feasible to prepare red labradorite with this treatment.
According to the LA-ICP-MS data in table 2, the copper concentration of these labradorite feldspar samples increased from 0.1–18.6 ppmw to 217.7–1086.0 ppmw with treatment. Therefore, we speculate that these two fluorescence emission peaks are related to copper. Based on previous studies of copper, CuO, Cu2O, and CuZr2(PO4)3 (Boutinaud et al., 1992, 1995; Lutz et al., 1997; Puppalwar et al., 2011), we attribute the strong fluorescence emission peak near 394 nm to Cu+ ions, and the fluorescence emission peak near 554 nm to the Cu+-Cu+ dimers (Zhou et al., 2022a,b,c). The dimers form when the Na+ sites in labradorite are simultaneously occupied by two Cu+ ions with a rather short Cu-Cu distance of 2.40 Å—i.e., below the interatomic distance in copper metal (2.56 Å). In other words, the typical fluorescence spectra or characteristics that are used to identify our diffusion-treated red labradorite feldspar can be explained by the residual Cu+ ions within the feldspar.

Appearance, Fluorescence Spectra, and Copper Concentration of Light Yellow Andesine Feldspar. According to the LA-ICP-MS data in table 2, the untreated feldspar crystals from Inner Mongolia are classified as andesine, and the average mass concentration of element copper is 0.3–1.1 ppmw. The andesine feldspar crystals are transparent and light yellow (figure 6 inset) before the high-temperature copper diffusion treatment. The weights of the Inner Mongolian andesine feldspar rough samples ranged from 0.46 to 1.78 ct. Figure 6 shows the fluorescence spectra of the untreated andesine feldspar samples, which exhibit weak fluorescence emission in the 400–410 nm wavelength range under 320 nm excitation. The intensity of the fluorescence emission peak is 190–550 cps. It must be mentioned that there is another set of fluorescence emission peaks at ~350 nm. These weak fluorescence emissions may also be related to the presence of copper or other trace elements contained in the feldspar crystals, but this has not been verified.

Changes in Andesine Feldspar Appearance, Fluorescence Spectra, and Copper Concentration After Copper Diffusion. The insets in figures 6 and 7 show the appearance of four andesine feldspar samples before and after copper diffusion treatment, respectively. The color of these samples changed from light yellow to light red. The fluorescence spectra in figure 7 clearly show that all of the copper-diffused red andesine feldspar samples also exhibit two typical fluorescence emission peaks under 320 nm excitation. Instead, the fluorescence emissions at ~350 nm in untreated andesine samples disappeared. The wavelength of the first emission peak is 397.4–398.6 nm with an intensity of 3942–6486 cps, while the wavelength of the second emission peak is ~554 nm with an intensity of 243–495 cps. These spectral parameters are summarized in table 3. According to the LA-ICP-MS data in table 2, the copper concentration of these andesine feldspar samples increased from 0.3–1.1 ppmw to 599.8–789.5 ppmw following copper diffusion treatment. The related changes in copper concentration and fluorescence of group B andesine feldspar subjected to copper diffusion are essentially the same as those of group A labradorite feldspar. We found that the strong fluorescence emission of diffusion-treated red labradorite and andesine feldspar is potentially a key indicator of treatment, since the strong fluorescence is difficult to attenuate through post-annealing treatment.
Appearance, Fluorescence Spectra, and Copper Concentration of Natural Labradorite Sunstone from Oregon and Ethiopia. To determine whether the fluorescence characteristics observed in our copper-diffused red feldspar samples matched those of natural red labradorite sunstone from different localities, we systematically characterized untreated red labradorite sunstone from three different mines in Oregon and also from Ethiopia. To our knowledge, the typical fluorescence spectra or characteristics of copper-diffused red feldspar under 305–335 nm UV light excitation have not been previously published or reported.
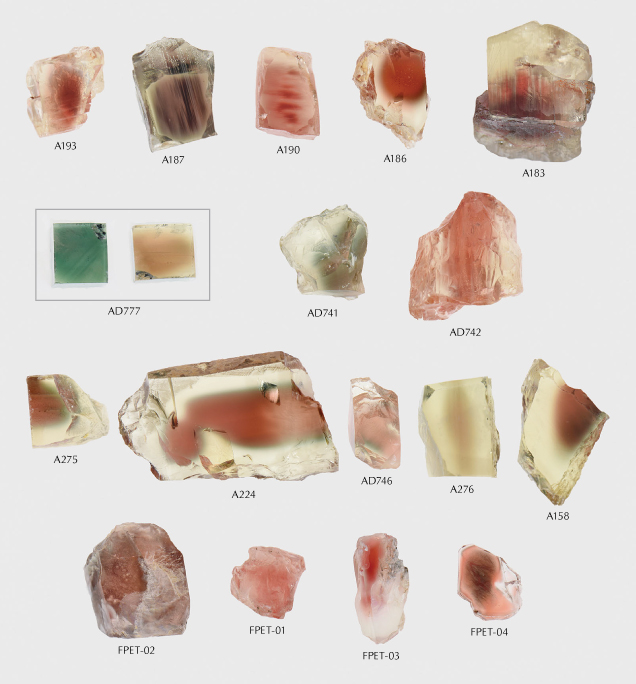
Figure 8 shows the appearance of the selected 17 natural labradorite sunstones from different localities, arranged by decreasing fluorescence intensity from left to right in each row. The natural labradorite sunstones show various shades of red, and a small number of samples appear green (AD741) or display red-green dichroism (AD777). The color distribution of these natural labradorite sunstones analyzed is mostly uneven, but all of them exhibit good transparency.

All the natural labradorite sunstones exhibit considerably weaker fluorescence emission than the copper-diffused red labradorite feldspar samples (figure 9). The emission wavelength of the labradorite sunstones from Ponderosa is 388.2–389 nm, and that of the labradorite sunstones from Dust Devil is 392.1–393.5 nm. The range of peak intensity values at 554 nm of the labradorite sunstones from Ponderosa is only 433–877 cps, and that for the labradorite sunstones from Dust Devil is only 292–568 cps. In terms of the fluorescence spectra, the natural labradorite sunstones from the Sunstone Butte mine in Oregon and the Afar region of Ethiopia are somewhat like those of the nearly colorless labradorite feldspar samples in figure 2. The emission wavelength fluctuates in a relatively wide range (394.2–413.0 nm), and the peak intensity is also very weak (74–367 cps). These spectral parameters are also summarized in table 3. For the natural labradorite sunstones from the Sunstone Butte mine with dichroism (AD777), the fluorescence emission spectra of both the red and green orientations were tested, and the results were basically the same. The inert fluorescence of sample AD777 under 320 nm UV light is shown in the inset of figure 9. The natural labradorite sunstone samples in figure 8 are arranged by the decreasing peak intensity in each row, but no direct correlation was found between fluorescence emission intensity and the color appearance of natural labradorite sunstone.
The fact that natural labradorite sunstone occurs in Oregon is recognized by gemological research institutions around the world. By comparing the fluorescence spectra of the copper-diffused labradorite feldspar samples with the natural labradorite sunstone, we have observed that strong fluorescence is potentially key evidence for copper diffusion treatment in labradorite, since none of the natural labradorite sunstones tested exhibited fluorescence of the same strength.
According to the LA-ICP-MS data in table 2, the copper concentration of natural labradorite sunstones from different localities is 0.1–182.6 ppmw, which is lower than that of the copper-diffused red labradorite feldspar samples (217.7–1086.0 ppmw). The comparative analysis of the natural labradorite sunstones reveals that the fluorescence intensity apparently has no direct correlation with the copper concentration measured by LA-ICP-MS, because the valence state of copper was not considered. Therefore, we infer that the weak fluorescence is due to the fact that copper in natural labradorite sunstone is mainly present in the metallic zero-valent form (Cu0), since copper microinclusions (such as copper flakes) have been observed in many natural labradorite sunstones.
Table 3 summarizes the fluorescence emission wavelengths and peak intensities of the samples tested, including the natural labradorite sunstones from different localities and the copper-diffused red labradorite and andesine feldspars we experimentally produced.

Figure 10 presents a plot of fluorescence intensity vs. major peak wavelength of Cu+ using the data summarized in table 3, which displays an obvious separation between the natural labradorite sunstones before and after copper diffusion treatment. The discrimination between natural labradorite sunstone (weak fluorescence) and copper-diffused labradorite feldspar (strong fluorescence) is very high. Moreover, the corresponding coordinates of all the copper-diffused red andesine feldspars are distributed in the area of strong fluorescence. Although the number of natural labradorite sunstones we analyzed is limited, they represent most of the localities worldwide, and we introduced red labradorite and andesine feldspar samples diffused in our laboratory as the control group. Therefore, we believe that the proposed identification method using fluorescence spectroscopy holds promise for copper diffusion treatment of labradorite.
CONCLUSIONS
In this study, we applied a copper diffusion treatment to nearly colorless or light yellow labradorite and andesine feldspars in order to modify their colors to mimic natural red/green labradorite colors. We found that the typical fluorescence emissions at 390–398 nm and at approximately 554 nm were stronger in the treated material. By comparing the fluorescence spectra of the labradorite and andesine feldspars before and after copper diffusion, the presence of strong fluorescence (320 nm excitation) was verified as key evidence of treatment in this study. Moreover, the natural labradorite sunstones from Oregon and Ethiopia analyzed exhibit weak fluorescence (320 nm excitation), similar to the untreated nearly colorless labradorite.
As a non-destructive testing method, fluorescence spectroscopy can be quickly applied to the questionable stones to identify very high emission counts and to decide if detecting high copper levels with LA-ICP-MS is warranted. Admittedly, more stones need to be tested to further validate the reliability and accuracy of this method. We still believe this new identification method will help to verify the authenticity of material from Tibet or other regions as new localities of natural sunstone, increasing confidence for consumers in purchasing these gems.



