Natural-Color Pink, Purple, Red, and Brown Diamonds: Band of Many Colors

ABSTRACT
Diamond is one of Earth’s most extraordinary materials. It represents the pinnacle for several material and physical properties. As a gem, however, it is the near-perfect examples—diamonds attaining the D-Flawless distinction—and those with imperfections resulting in a vibrant or surprising color that create the most enduring impressions. Fancy-color natural diamonds are among the most highly valued gemstones due to their attractiveness and great rarity. The 18.96 ct Winston Pink Legacy, with a color grade of Fancy Vivid pink, recently made history by selling at over $50 million, its $2.6 million per carat price an all-time high for a pink diamond (Christie’s, 2018).
INTRODUCTION
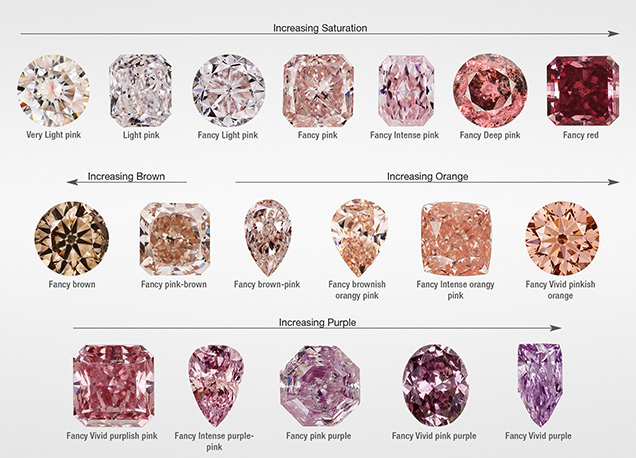
This article is the third in a series of studies on natural colored diamonds: Breeding et al. (2018) discussed the color origin of green diamonds, and Eaton-Magaña et al. (2018) dealt with blue/gray/violet diamonds. Here we summarize the color origin of orangy pink, purplish pink to pinkish purple, brownish pink to pinkish brown, pink, red, purple, and unmodified brown diamonds. Although this article covers a wide variety of color descriptions compared to, for example, the first article in the series, which was limited to natural diamonds with only green as the dominant description, the color descriptions are joined here, as the vast majority of them (which will be quantified below) contain the 550 nm band as a prevalent absorption feature. For the sake of simplicity, this broad range of color descriptions (e.g.,figures 1 and 2) will generally be shortened to “pink” in this article.
Previously, several aspects of gem-quality “pink” diamonds have been addressed, such as their gemological characteristics (e.g., King et al., 2002); their defects and associated spectroscopic features (e.g., Titkov et al., 2008; Deljanin et al., 2008; Fisher et al., 2009; Gaillou et al., 2010; Stepanov et al., 2011; Byrne et al., 2012a,b; Titkov et al., 2012; Gaillou et al., 2012; Howell et al., 2015); and reports on notable “pink” diamonds (Smith and Bosshart, 2002; King et al., 2014). Here we review the published literature and supplement it by compiling results obtained from 90,000+ natural diamonds recorded in the GIA internal database.
Our purpose is to provide a detailed account of the gemological and spectroscopic characteristics of natural “pink” diamonds, principally colored by an as-yet-unidentified “550 nm” absorption band, in order to help the trade better understand how these beautiful diamonds with extremely valuable colors originate.
CAUSES OF COLOR
All colored diamonds submitted to GIA are subject to a variety of spectroscopic analyses. The analytical techniques and specific details of the instrumentation and methods used are summarized in Breeding et al. (2018; see supplementary table S-1).
“Pink” color in diamonds spans a wide range in the GIA color description terminology (figure 2; King et al., 1994; King, 2006). In this article, we include all natural fancy-color diamonds with pink, purple, or red as the dominant hue (i.e., the final name in the color description) in addition to unmodified brown. Diamonds with yellow-brown or orange-brown color will be discussed in an upcoming article. In the full dataset of 90,000+ “pink” diamonds seen at GIA between January 2008 and December 2016, the highest percentage were unmodified pink (40%), followed by pinkish purple to purplish pink (28%), brownish pink to pinkish brown (17%), and orangy pink (10%); see the color distribution in figure 3. The rarest colors were unmodified brown (3%); purple with brown or gray modifiers (1%); unmodified red (0.5%); red with brown, purple, or orange modifiers (0.4%); and, least of all, unmodified purple (0.05%).

In the description of color grades, it is more difficult for the eye to interpret subtle hue differences for very light-colored diamonds than it is for more saturated diamonds (King et al., 1994; King, 2006). For the lightest color grades, therefore, fewer hue names are used and the color description spans a wider portion of the hue wheel. Among unmodified pink diamonds, 54% were Faint, Very Light, or Light in color (figure 3, right).
Below we chronicle the main causes of color in the form of various structural defects, through which “pink” diamonds can be divided into different groups. Various colors and representative visible-near infrared spectra are shown in figures 2 and 4, respectively.

Colored Deformation Lamellae. In many brown, pink, and purple diamonds, the color is concentrated within parallel narrow bands (referred to as “brown graining,” “pink graining,” or glide planes, or alternatively as a more generalized term of colored lamellae). When manufactured as cut stones, these are typically oriented to minimize the face-up appearance of the lamellae, resulting in an apparent even color distribution through the table facet. Since the lamellae are along the {111} plane, these pink diamonds are generally oriented such that the table facet is {100} (J. Chapman, pers. comm., 2018). Micro-scopic observation of the lamellae is best accomplished looking through the pavilion facets of the diamond.
These colored lamellae are caused by shear stress and natural plastic deformation (figure 5, A and B). Deformation to form these lamellae only occurs during a diamond’s extended residency at high temperature ( > 900°C; DeVries, 1975; Brookes and Daniel, 2001), either in the mantle or during kimberlite eruption to the earth’s surface. These deformation lamellae have been attributed to slip/glide planes (dislocation movement) and to micro-twins (Mineeva et al., 2009: Gaillou et al., 2010; Titkov et al., 2012; Howell et al., 2015). In many diamonds these deformation lamellae are closely associated with brown, pink, or purple color; these colors can also appear evenly through the entire diamond or irregularly as distinct patchy areas (figure 5, C and D). The structural defects responsible for the different color contributions in pink, brown, and purple diamonds (i.e., the “brown absorption continuum,” the 550 nm absorption band, and NV0/– centers) are discussed in later sections.

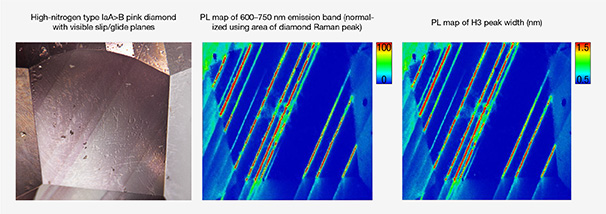
Viewed with a microscope, the two types of lamellae appear distinct, and at GIA these are referred to as either slip/glide planes or graining. They also appear to correspond with the ratio of A centers (nitrogen pairs) and B centers (four nitrogen atoms around a vacancy) in the diamond (Gaillou et al., 2010). As diamonds reside in the mantle, or are exposed to higher temperatures, the A-aggregates decrease as they combine to form B-aggregates (Taylor et al., 1990). “Pink” diamonds where A-aggregates outnumber B-aggregates (i.e., type IaA > B, such as some Siberian diamonds; Titkov et al., 2008) show colored lamellae that appear distinctly different from “pink” diamonds where B-aggregates are greater than A-aggregates (i.e., type IaA < B, such as Argyle diamonds; Howell et al., 2015). In type IaA > B “pink” diamonds (designated below as Group 2), the colored lamellae appear distinct and straight along the {111} planes, and the pink color is restricted to these colored lamellae (Gaillou et al., 2010). Between these lamellae, the diamond is colorless (e.g., figure 5A). GIA often refers to these lamellae as “slip/glide planes” to distinguish them from the other type of colored lamellae. In type IaA < B “pink” diamonds (designated below as Group 1; figure 5B), the pink color is not restricted to the lamellae and does not appear as straight lines. GIA commonly refers to this as “graining,” although these distinctions in terminology have not been defined within the gemological literature.
In faceted diamonds, the colored lamellae of type IaA < B “pink” diamonds can appear more subtle than the apparent contrast in color within type IaA > B “pink” diamonds (compare figure 5A with 5B). Recent research (see box B) shows that these classifications are not quite so distinct for diamonds that are low in nitrogen, and the visual distinction between colored “graining” and “slip/glide planes” becomes challenging.
In contrast, the majority of type IIa “pink” diamonds, and some pure type IaB diamonds, do not show visible evidence of colored lamellae (either as “graining” or “slip/glide planes”), and the “pink” color is uniformly distributed. However, type IIa “pink” diamonds do occasionally exhibit graining (appearing as colorless, brown, or pink) similar to that seen in type IaA < B diamonds.
Brown General Absorption. Brown color is quite common in natural diamonds, and a brownish tinge is reportedly observed in 98% of all as-mined diamonds, whether or not they are deemed gem-quality (Dobrinets et al., 2013). While brown diamonds are abundant, most are either not faceted or not submitted for grading reports unless there is another contributing color such as pink, orange, or yellow or if they can be faceted in such a way as to receive a grade on the D-to-Z scale (King et al., 2008). As mentioned previously, only 3% of the 90,000+ samples studied here had an unmodified brown color.
The vast majority of brown diamonds have their color ascribed to vacancy clusters (i.e., clusters of vacant carbon-atom positions; see Hounsome et al., 2006; Fisher et al., 2009) that are created through natural plastic deformation. These clusters are generally thought to contain approximately 40–60 vacancies, and the dissociation (generally through high-pressure, high-temperature HPHT annealing) of these vacancy clusters leads to the removal of brown color. These vacancy clusters create a so-called brown absorption continuum (Fisher et al., 2009; Dobrinets et al., 2013) that causes a gradually increasing absorption across the visible spectrum toward lower wavelengths (figure 4, spectrum D). This brown absorption continuum can coexist with other color centers (e.g., figure 4, spectrum C).
Brown coloration (by itself or in combination with other color centers) can also have other origins, including high concentrations of isolated substitutional nitrogen, CO2, and micro-inclusions (Hainschwang et al., 2008; Dobrinets et al., 2013). However, these are not as commonly observed as those diamonds with the brown absorption continuum.
550 nm Visible Absorption Band. The most common cause of “pink” color is a broad absorption band centered at around 550 nm (Orlov, 1977; figure 4, spectrum B). The defect responsible for this visible absorption band has not yet been identified, but it has been correlated with plastic deformation (Gaillou et al., 2010; Howell et al., 2015). In conjunction with the 550 nm band is a smaller, narrower band within the UV range centered at ~390 nm (Fisher et al., 2009; Byrne et al., 2012a).
In “pink” type Ia diamonds, nitrogen often creates other visible absorption features that are detected in the visible absorption spectra and can affect the observed color. The N3 defect (zero phonon line, or ZPL, at 415.2 nm) and the H3 defect (ZPL at 503.2 nm) along with their vibronic structure (sidebands) create additional absorption at lower wavelengths (figure 4, spectrum A) that can alter the observed color.
Nitrogen-Vacancy Centers. “Pink” diamonds naturally colored by NV0/– centers are extremely rare; however, these centers are almost exclusively the cause of color in treated and synthetic “pink” diamonds. Absorption by the nitrogen-vacancy centers (the ZPL for the neutral NV center is at 575 nm, while the negative NV center is at 637 nm) along with their sidebands creates the absorption that produces the pale, uniform “pink” color in these very rare type IIa diamonds (figure 4, spectrum E). They are often referred to as “Golconda pink” diamonds (Fritsch, 1998). The 34.65 ct Fancy Intense pink Princie is a famous example of “Golconda pink,” and it sold for more than $39 million at auction in 2013 (Christie’s, 2013). While the Princie originates from the historical mining region of Golconda in India, today the term “Golconda” is generally associated with this cause of color and not with any geographical link to the ancient mining region.
OCCURRENCE AND FORMATION
The formation mechanism for “pink” diamonds in the earth varies significantly depending on the defects responsible for the color. Historical sources include India, Brazil, Indonesia (Borneo), and southern Africa. But before the Argyle mine in Australia opened in 1983, there was no stable supply of “pink” diamonds, and many of those currently available come from Argyle (figure 6; Hofer, 1985; Shigley et al., 2001; Shirey and Shigley, 2013). More recently, mines in the Siberian and Arkhangelsk regions of Russia have been documented as producing pink to purple-pink diamonds (Titkov et al., 2008; Smit and Shor, 2017). Finding new deposits with “pink” diamond production is important to the trade, since the final year of planned operation at the Argyle mine is 2020 (Shor, 2018).

“Pink” Diamonds with 550 nm Absorption Band. Occurrence. Nitrogen content and the extent of nitrogen aggregation in the atomic structure, and by inference the diamond’s geographic origin, leads to very different observations of how “pink” color due to the 550 nm band is distributed within the diamond. Sources for Group 1 “pink” diamonds (type IaA < B, with wavy graining) include the Argyle mine in Australia and alluvial deposits in Venezuela. Group 2 “pink” diamonds (type IaA > B, slip/glide planes) are principally mined in southern Africa, Canada, and Russia (Howell et al., 2015).
An abundance of locality data is available on type Ia “pinks” but not for type IIa “pink” diamonds. There are several observations of type IIa “pinks” that coincide with Argyle “pinks” such as color range, the presence of wavy graining in some type IIa diamonds, and some photoluminescence peaks that appear in type IIa, IaB, and IaA < B “pinks.” However, reports regarding the output of the Argyle mine largely exclude type IIa diamonds (J. Chapman, pers. comm., 2018). Type IIa pink-brown diamonds are known from localities that also yield so-called superdeep diamonds from > 250 km in the earth’s mantle (Smith et al., 2017). Pink-brown type IIa diamonds with superdeep mineral inclusions (i.e., minerals that are only stable, or could only form, under high-pressure conditions) have been documented in GIA’s New York laboratory, but their geographic source location is unknown. One of the few superdeep IIa “pink” diamonds for which GIA does have locality information is a 23 ct rough pink type IIa diamond found at the Williamson mine (Tanzania) in November 2015 (Smith et al., 2017; Petra Diamonds, https://www.petradiamonds.com/about-us/our-heritage). Fisher et al. (2009) also cited the Williamson mine as a source of type IIa pinks, though production numbers are unknown. The Williamson mine has also been a source of high-nitrogen type Ia “pink” diamonds (Gaillou et al., 2012).
Although “pink” diamonds are known from several deposits around the world, they remain a very small percentage of the overall production from any particular deposit. The Argyle mine is well known as a source for “pink” diamonds, but these account for much less than 0.1% of the overall production, where 72% of the production is brown and 27% is colorless to yellow (Shigley et al., 2001; figure 6). At the Lomonosov deposit in northwestern Russia, only 0.04% of production is fancy-color diamonds (purple, pink, violet, green, yellow, and brown; Smit and Shor, 2017). Some parcels from the Mir deposit in Siberia contain 1–6% pink to purple diamonds, although no overall production data were available (Titkov et al., 2008 and references therein).
Formation. Pink-brown color is often associated with deformation lamellae in the diamond, and the geological process responsible for this deformation is often mountain building (Stachel et al., 2018). A geologically modern example of mountain building is the Himalayan range in Asia, which is increasing in elevation due to collision between the Eurasian and Indian tectonic plates. The remnants of mountain ranges that developed during collisional processes millions to billions of years ago, and have since been eroded away, are called “orogens.” During continental collision, rocks become highly deformed with such severe mineral recrystallization that any diamonds and other minerals in the rock can take on a “flow” texture.
The Argyle mine, the most famous supplier of occasional pink and red diamonds, occurs in one such area that experienced ancient continental collision, the Proterozoic Halls Creek orogen (1.8 Ga; Tyler and Page, 1996). Argyle diamonds formed in close association with these collisional processes (Richardson et al., 1985), and after their formation resided near the base of the lithosphere (Stachel et al., 2018). It is in this high-temperature, high-deformation environment near the convecting mantle that the pink-brown-red color in these diamonds likely originated.
The Lomonosov mine, which produces pink-purple-brown diamonds, also lies within an area that experienced ancient subduction and continental collision—around 1.9 billion years ago—and is known as the Lapland-Kola orogen (Daly et al., 2006). Although the Lomonosov diamonds have not been dated, it is very possible that they formed in association with subduction, or experienced deformation related to the subduction-collision events, during their residence at depth in the lithospheric mantle (Smit and Shor, 2017).
Diamonds’ presence within an orogenic belt does not necessarily imply high deformation that could result in pink-brown colors. The Ellendale mine is in the King Leopold orogen, a geologic region that experienced mountain building 545 million years ago along the southwest margin of the Kimberley craton in Australia. Although diamonds from this deposit are famous for their fancy colors, they are yellow (not the pink-brown colors typically associated with deformation) and the defects responsible for their yellow color are the very common “cape” absorption series (N3 and N2 defects). Additionally, cathodoluminescence images of the internal growth features in these diamonds are not dominated by deformation lamellae (Smit, 2008). Together, the bodycolor and lack of deformation features suggest that the 1.4 billion-year-old Ellendale diamonds survived in the lithospheric mantle through the Proterozoic orogenic event (until their eruption at 20 Ma) without being significantly deformed, implying that the King Leopold orogenic event was limited to the earth’s crust.
Pink Diamonds with NV0/– Centers. Occurrence. Although pink diamonds colored by NV0/–centers (i.e., “Golconda pinks”) are associated with the ancient diamond mining and trading center of Golconda in India (now Hyderabad), there are no known reliable modern sources in this region. Natural diamonds with sufficient concentrations of NV0/– absorption to contribute to the pink color are extremely rare. The “Golconda pink” diamonds examined in GIA laboratories (0.6% of 1,000 randomly selected “pink” diamonds; see the Absorption Spectroscopy section for further details) do not have any geographic source information.
Formation. The combination of geologic conditions necessary to create and preserve NV0/– centers in natural diamonds is exceedingly uncommon. NV0/– centers form when vacancies in the diamond lattice are trapped next to isolated substitutional nitrogen. Although these defects are rarely preserved in nature, they are relatively easy to create in a laboratory. Three rare conditions are needed to produce natural “Golconda pink” diamonds.
First, these diamonds need low nitrogen concentrations such that when any nitrogen present in the lattice combines with vacancies upon eventual annealing, no infrared-active nitrogen is detected. This would classify the diamond as rare type IIa, which according to a recent survey make up only 1.3% of diamonds submitted to GIA for grading (out of 3.5 million diamonds; Smith et al., 2017).
Secondly, vacancies need to be present in the diamond lattice. Vacancies in the lattice are created either through irradiation or deformation. Radiation exposure is rare in the mantle, and typically only occurs once the diamond is erupted to the earth’s surface and has been in contact with crustal rocks or fluids that contain sufficient concentrations of elements such as uranium, thorium, or potassium (Breeding et al., 2018). Since high temperatures above 600°C are needed to combine vacancies with nitrogen, it is more likely that deformation processes in the mantle are responsible for vacancy creation. For example, brown coloration due to vacancy cluster formation often originates from deformation of diamond in the mantle (Fisher et al., 2009).
Thirdly, in order to preserve isolated nitrogen for annealing, and prevent any NV centers from annealing further to form A and B nitrogen centers, the diamonds must not have experienced high temperatures of greater than 900°C for more than a few million years (Smit et al., 2016, 2018).
The exact geological environment in the deep earth where all these conditions are met is not clear. It is possible that some “Golconda pink” diamonds are superdeep, since type IIa diamonds often have a sublithospheric origin (depths > 300 km; Smith et al., 2016, 2017), and these superdeep diamonds often exhibit deformation features. For example, brittle fractures along the {110} crystallographic planes as well as polygonized dislocation networks have been observed in deformed superdeep diamonds (Smith et al., 2016, 2017). Deformation processes responsible for these features could be related to mantle convection or deep subduction processes. However, sublithospheric mantle temperatures are in excess of 1400°C, and it is not clear how isolated nitrogen and NV0/– would survive at these temperatures without being annealed to more aggregated nitrogen, which is common in superdeep nitrogen-bearing diamonds.
LABORATORY GRADING
Stones weighing less than 1.0 ct comprised 83% of GIA intake of the 90,000+ natural “pink” diamonds in this study, and more than half (56%) weighed less than 0.5 ct (figure 7). This chart clearly shows that while the total number of “pink” diamonds appears to be quite large—compared to 15,000+ in the blue/gray/violet group (Eaton-Magaña et al., 2018) and 50,000+ in the green group (Breeding et al., 2018)—all colored diamonds submitted to GIA annually represent approximately 3% of overall intake. The vast majority are also quite small. Noticeable spikes in quantity are observed near important carat-weight thresholds (1.0, 1.5, 2.0, 3.0 ct, etc.), and many have a light color (figure 3, right).

This chart also serves to illustrate that while cutters make faceting and polishing decisions to maximize the face-up color and appearance, weight thresholds are often important considerations as well. Diamonds with purplish pink coloration are much more prevalent at weights below 1 carat, while those with brown coloration are much more prevalent above 1 carat (figure 7), likely because it is only worthwhile to submit brown diamonds in larger sizes.
Although colored diamonds are often fashioned as fancy shapes to enhance their face-up color, rounds represent a slight plurality (24%) of the 90,000+ “pink” diamonds (figure 8), followed by pears (20%), rectangles (16%), and cushions (13%) and others. While the fancy shapes are generally evenly distributed across the range of weights and color saturations, the rounds are predominantly seen among the smaller and lighter-color diamonds. Of all fancy-color “pink” rounds, 90% are less than one carat and 42% are Faint, Very Light, or Light.

ABSORPTION SPECTROSCOPY
To investigate the distribution of the major causes of color, we studied the visible absorption and IR absorption spectra of 1,000 natural brown, pink, purple, and orangy pink diamonds. These were randomly selected as representative of the 90,000+ samples in our internal GIA database. This subset of data was used to generate the charts provided in figures 9–15.


Absorption spectroscopy measurements are nondestructive and indicate the major defects and impurities within a diamond by passing light through the stone and measuring the wavelengths (energies) absorbed by the impurities and defects present. Defects that cause absorption within the visible spectrum (between 400 and 700 nm) contribute to the bodycolor of the diamond. Other defects that are detected only through infrared (IR) absorption spectroscopy do not give information about the color-contributing defects but provide an indication of whether a diamond is natural, synthetic, or treated.
For this 1,000-diamond subset, we observed that 992 (99.2%) contained the 550 nm band. Six were colored by NV0/– defects (0.6%); this percentage indicates the extreme rarity of “Golconda pink” diamonds even among this restricted population of “pink” diamonds. The remaining two of the 1,000 diamonds in this subset were unmodified brown diamonds that did not show an observable 550 nm band, while the other brown diamonds showed a slight 550 nm band that did not cause sufficient absorption to affect the color (figure 4, spectrum D). The majority of this section will focus on the optical and infrared absorption spectra of diamonds colored by the 550 nm absorption band, and the diamonds containing NV0/– defects will be briefly discussed at the end.
Vis-NIR Absorption Spectroscopy of 550 nm “Pink” Diamonds. As the vast majority of these diamonds were principally colored by the 550 nm band, we looked for subtle distinctions between the Vis-NIR absorption spectra to chronicle their differences. As such, the Vis-NIR absorption spectra of most diamonds were distinguished by the presence or absence of the N3 and H3 centers, so we could gauge whether these had any effect on the resulting color grade. Figure 9 shows the effect of these various Vis-NIR absorption spectral features on the resulting hue and tone. Figure 9A indicates that the addition of N3 and H3 does not appear to correlate with the observed hue (that is, the proportion of diamonds with H3 and/or N3 does not significantly change between those with brown-pink, pink, and purple-pink designations). Unmodified purple and red diamonds deviate from this observation and are discussed in box A. Additionally, orange-pink diamonds showed a slightly higher percentage with H3/N3 centers; when these were present, the increased absorption of blue light shifted the perceived color to orange. However, the presence of N3 and H3 centers did have a dramatic effect on the tone of the color (figure 9B). In most cases, those diamonds with faint colors only have a detectable 550 nm band. For the majority of diamonds with a fancy or deeper tone, the 550 nm band is enhanced by the addition of the N3/H3 center (or the presence of both features).
The spectra of 50 “pink” diamonds with the 550 nm band were analyzed to determine the full width at half maximum (FWHM) of the band and the center wavelength, as minor variations in the peak width and position of this band can affect the color. The center wavelength of the absorption band is nominally at ~550 nm, but performing peak fitting using a mixed Gaussian/Lorentzian equation shows that the center wavelength can vary from 545 nm to 565 nm. Meanwhile, the width of the absorption band can vary from 60 nm to 100 nm. Fitting the 550 nm absorption bands from diamonds of various hues showed that the orangy pink to pink diamonds generally had a lower center wavelength (545–550 nm) and a narrower width (60–80 nm) than purple-pink diamonds (555–560 nm and 70–100 nm, respectively); for example, figure 9C compares the Vis-NIR absorption spectra of a purple and pinkish orange diamond. The slight shift of the 550 nm band away from the orange to red portion of the visible spectrum (for orangy pink diamonds) or away from the blue (for purple-pink to purple diamonds) can contribute to the transmission window that leads to the resulting bodycolor. A notable percentage of pink to purple-pink (~20%) also show a series of oscillations overlaid on the 550 nm band at ~600 nm (figure 4, spectra B and C; box B).
Fourier-Transform Infrared (FTIR) Absorption Spectroscopy of 550 nm “Pink” Diamonds. Nitrogen Aggregation. Within the 1,000-diamond subset, 992 contained the 550 nm band, as stated above. Of these, 243 (24%) were type IIa based on IR absorption spectra along with the six diamonds colored by NV0/– centers (i.e., “Golconda pinks”), for a total of 249 type IIa diamonds. The remaining 751 diamonds all contained aggregated nitrogen (figure 10A shows a variety of the IR spectra obtained for “pink” diamonds along with the corresponding A and B nitrogen aggregate concentrations, and figures 10B and 10C show the overall distribution of diamond type along with the origin of color and color description). Those 170 termed as type Ia (17%) had saturated nitrogen concentrations in their IR absorption spectra, and the specific A and B nitrogen aggregates were too great to be resolved with our instrumentation and thus could not be calculated. The remaining 581 diamonds had sufficiently low nitrogen to determine the aggregate concentrations (Boyd et al., 1994, 1995). Of those, 396 were type IaA < B (40%) followed by 125 type IaA > B (13%), 31 pure type IaA (3%), and 29 pure type IaB (3%).
Figure 10B shows that the type IIa “pink” diamonds were overwhelmingly colored by the 550 nm band alone and generally did not include the additional H3 and N3 centers. This is not surprising, as type IIa diamonds do not have sufficient nitrogen concentration to generate nitrogen-related defects that could be detected by visible absorption and affect the color.
Among the diamonds with nitrogen aggregates, many were colored by H3 and/or N3 that appeared to impact the color saturation more than the hue, as indicated by comparing figure 9A and 9B. Among type IaA diamonds, very few showed either H3 or N3 centers. In contrast, a majority of type IaA < B diamonds had both the N3 and the H3 in addition to the 550 nm band.
The observation that diamonds with higher amounts of B-aggregates also showed a higher incidence of H3 and N3 centers is not surprising given the evolution of these various defects within diamond. A very young diamond will show only isolated nitrogen. As time progresses, these nitrogen atoms combine to create more and more complex defects. The A-aggregates are composed of two nitrogen atoms; the H3 defect contains two nitrogen atoms plus a vacancy; the N3 defect includes three nitrogen atoms with a vacancy; and the B-aggregate includes four nitrogen atoms plus a vacancy. As the A-aggregate/B-aggregate ratio shifts toward higher amounts of B, other defects (such as H3 and N3) will be produced as well (Dobrinets et al., 2013).
Concentrations of A and B Nitrogen Aggregates. The ratio between A and B nitrogen aggregates can be used to assess the time and/or temperature of a diamond’s residence deep within the earth (Leahy and Taylor, 1997). Even if the total nitrogen concentration is different between diamonds, the transition from A-aggregates to B-aggregates increases at higher temperatures and longer timescales. If a population of diamonds has similar A-to-B ratios, they likely resided under similar time and temperature conditions, increasing the likelihood that they originated from the same source.
As mentioned previously, 581 of the “pink” diamonds from the 1,000-diamond subset had IR spectra from which the concentrations of A- and B-aggregates could be calculated (in atomic parts per million, or ppma; figure 11). These were calculated individually by baseline correcting each spectrum, and then calculating A- and B-aggregates using a spreadsheet provided by Dr. David Fisher (DTC Research Center, Maidenhead, UK) using absorption coefficients from Boyd et al. (1994, 1995). The majority of the 581 diamonds contained more aggregated nitrogen as B centers (type IaA < B; figure 11).

In the inset of figure 11, most of the datapoints are clustered near the green guide line (i.e., the calculated best-fit line for type IaA < B diamonds), indicating that A/B concentrations generally lie along that slope. Although the total nitrogen concentrations vary from a few ppm to a few hundred ppm, the conversion of A- aggregates to B- aggregates is roughly consistent for all values near the green slope line. This consistency strongly suggests that all of these diamonds experienced similar time/temperature conditions within the earth and potentially from the same mine. The sheer quantity of diamonds plotted here (compared with those scattered among the rest of the graph) imply that they come from the most abundant source—the Argyle mine—although they could also be from Namibia or Venezuela. Also plotted are the data of 67 diamonds, unrelated to this study, that are known to be sourced from the Argyle mine (unpublished GIA data, 2007). In contrast, pink to purple diamonds sourced from Siberia are predominantly type IaA > B, and two known Siberian samples with unsaturated IR spectra (Titkov et al., 2008) are also shown alongside the data from this study. This geographical distinction based on nitrogen aggregation has been documented before (Gaillou et al., 2010; Howell et al., 2015), and a good hypothesis of origin is possible, though not with any reliable certainty, as “pink” diamonds originate from very few sources.
Although this plot cannot include type IIa or saturated type Ia diamonds, it is consistent with prior reports (e.g., Howell et al., 2015) that “pink” diamonds clustered around the green slope line are mainly sourced from the Argyle mine, and these encompass a significant percentage of “pink” diamonds submitted to GIA. If we assume that the type IaB and IaA < B diamonds are generally from the Argyle mine and the other types, including the saturated type Ia diamonds, are sourced from other mines, then 425 of the 992 diamonds with the 550 nm band, a total of at least 43%, originated from Argyle.
The nitrogen aggregation results for these diamonds, shown in figure 11, are also split according to the color description of the “pink” diamond. This indicates several interesting trends. Among the type IaA < B diamonds (i.e., the left side of the diagonal in figure 11), most purple-pink to pink-purple samples are congregated in the portion that indicates low nitrogen. Also, the orangy pink diamonds are almost exclusively contained among the type IaA < B population, while a single orangy pink type IaA sample is represented elsewhere on the plot (see also figure 10C).
Among type IaA > B diamonds (i.e., the right side of the diagonal in figure 11), the nitrogen concentrations are higher, with many showing A-aggregate concentrations of 300 ppm or more. Also, many of the unmodified pink diamonds are nearly pure type IaA, while the purple-pink diamonds have a greater concentration of B-aggregates, consistent with previous reports by Titkov et al. (2008).
Effect of Total Nitrogen Concentration. To visualize the effect that nitrogen concentration had on color hue, tone, and spectral features (figure 12), we grouped the A < B and A > B data of all 581 “pink” diamonds shown in figure 11 by the total aggregate concentration into five different ranges. The concentration range of each group was determined in order to create roughly equal groups.

With increasing nitrogen, we generally saw a decrease in purple-pink to pink-purple diamonds (figure 12A); this is likely due to a greater absorption of the H3 and N3 centers that reduced transmission of blue light. Additionally, among type IaA < B with increased nitrogen there was a greater proportion of unmodified brown diamonds. The other hues showed fluctuations with increasing nitrogen, but no clear trends. Again, orangy pink diamonds are essentially seen only among diamonds with A < B aggregates.
Across the range of total nitrogen, type IaA < B diamonds show more saturated colors (figure 12B) than type IaA > B that appears due to the higher incidence of the N3 and H3 centers (figure 12C and consistent with figure 9). For the highest range shown in figure 12C ( > 250 ppm total nitrogen), more than 80% of the type IaA < B diamonds show one or both of these centers. The additional absorption of the N3/H3 centers increases the overall absorption in these diamonds, leading to more saturated colors.
Other IR Features. Of the 1,000-diamond subset, half were randomly selected and surveyed for the presence in their spectra of the N3VH defect (3107 cm–1 center), the platelet feature, and “amber” centers.
The 3107 cm–1 peak was designated for many years as a “hydrogen-related defect,” and only recently was it ascribed to N3VH by Goss et al. (2014). N3VH was frequently observed in our sample suite, occurring in 74% of the “pink” diamonds surveyed. Of the diamonds without N3VH visible in the infrared spectrum, 87% were type IIa. The saturated type Ia group contained 80% of the diamonds that were rated as having a strong 3107 cm–1 peak. The type IaA > B and IaA < B groups showed no significant difference in occurrence of the N3VH defect or in the strength distribution.
The platelet feature is comprised of extended defects involving carbon interstitials (i.e., clusters of carbon atoms not in normal positions in the lattice) and nitrogen in thin layers thought to form as A-aggregates transition into B-aggregates (Humble, 1982; Speich et al., 2017). The position of the peak can vary from 1358 to 1380 cm–1 depending on the diameter of the platelet, where the lower wavenumber value corresponds to a higher platelet diameter (Speich et al., 2017). None of the type IIa and IaB stones in our dataset had a platelet peak present in the IR spectrum, which is to be expected due to their lack of A centers (Humble, 1982). The IaA, IaA > B, IaA < B, and Ia groups all showed a platelet peak occurrence more than 50% of the time, with the Ia group having an occurrence of 96%. The strength of the platelet peak correlated best with the concentration of B-aggregated nitrogen, consistent with previous studies (Woods, 1986; Speich et al., 2017). The type Ia diamonds had the highest average peak position (and therefore the smallest average platelet diameter) at 1369 cm–1, and the IaA < B group had the lowest average peak position (and therefore the largest average platelet diameter) at 1362 cm–1.
The “amber” centers are a series of broad bands related to diamonds with brown coloration and/or colored graining, occurring between 4000 to 4400 cm–1. It has been suggested that the “amber” centers are defective A-aggregates formed along plastic deformation planes (Massi et al., 2005). Two specific bands were surveyed for this study: the “amber” centers at 4065 and 4165 cm–1. The 4065 cm–1 “amber” center has been observed to be stable up to 1700°C, while the 4165 cm–1 “amber” center can survive heating until 1900°C (Eaton-Magaña et al., 2017). As these two features are stable to different temperatures, they are likely different centers. Previous reports have shown that the 4065 cm–1 “amber” center is more prevalent in diamonds of type IaA > B (Gaillou et al., 2010).
The IR spectra were categorized as having neither peak, one of these peaks, or both (figure 13). None of the type IaB stones and very few (14%) of the type IIa diamonds showed either the 4065 or the 4165 cm–1 peak, consistent with the model of the “amber” center being a defective A center (Massi et al., 2005). As the concentration of A-aggregates increased, the likelihood of one or both “amber” peaks increased as well; the type Ia diamonds showed a high percentage of “amber” centers, likely corresponding to the high concentration of A-aggregates within them (although the saturated spectra do not allow us to accurately make that determination).

Diamonds Colored by NV0/– Centers. Only six of the 1,000-diamond subset were colored by NV0/– centers, thus rendering a very small sample size and provide little more to illuminate beyond what has already been discussed in the Causes of Color section. As expected, all six showed type IIa IR absorption spectra that were featureless except for intrinsic diamond features. In the Vis-NIR absorption spectra, the six diamonds (three orangy pink, two pink, and one brownish pink) had NV0/– centers at 575 and 637 nm along with their accompanying sidebands, with the most prominent of those centered at ~520 and 620 nm (figure 4, spectrum E). Of these, four had detectable GR1 peaks and four showed the 595 nm center commonly seen in irradiated diamonds (Breeding et al., 2018). The presence or absence of these radiation-related peaks did not correspond with the graded color.
PL SPECTROSCOPY
Photoluminescence (PL) uses lasers of different wavelengths to produce emission spectra that reveal the optical defects present in a diamond. PL analysis is one of the most useful and sensitive techniques for defect characterization. Using PL spectroscopy, defects occurring at parts per billion (ppb) concentrations (and therefore not necessarily contributing to the diamond’s bodycolor) can easily be detected (Eaton-Magaña and Breeding, 2016).
Diamonds Containing the 550 nm Absorption Band. The PL spectra of diamonds colored by the 550 nm absorption band often show features that either contain vacancies or correlate with plastic deformation. For example, the 566 nm triplet is seen in pink to brown diamonds of both type Ia and IIa (i.e., those with and without colored graining; GIA unpublished data).
Common vacancy-related peaks include the H3 (NVN0 at 503.2 nm), H4 (NVN– at 496 nm), GR1 (V0 at 741.2 nm), and NV centers (NV0 at 575 nm, NV– at 637 nm; figure 14). The NV0/– centers are often present in diamonds containing the 550 nm band but not in sufficient concentrations to be detected in the Vis-NIR absorption spectra, and therefore they do not affect the color. Peaks at 657, 661, and 668 nm are often seen together in type IIa, IaA < B, and IaB “pink” diamonds; however, the defect structure of these peaks has not been identified. Box B further discusses the photoluminescence features within the graining and slip/glide planes that are observed in type IaAB diamonds.

The peak width of the diamond Raman line is affected by the local strain environment, and an increase in crystallographic strain leads to a broadening of the peak width (Grimsditch et al., 1978). The diamond Raman line has been shown to increase in width on pink grain lines in type Ia diamonds, demonstrating that the pink lamellae lie in planes of plastic deformation (Gaillou et al., 2010).
In contrast, type IIa “pink” diamonds rarely show colored lamellae and instead are evenly colored. In type IIa diamonds showing a uniform pink coloration, this relationship of strain versus pink color is not so easily visualized. Therefore, we plotted the GR1 width (i.e., increasing strain) of 239 type IIa “pink” diamonds from the 1,000-diamond subset against pink color intensity (figure 15). This plot shows that in type IIa diamonds, an increase in strain (represented by the peak width of the GR1 center) corresponds to an increase in pink color saturation in type IIa diamonds, despite the lack of easily distinguishable strained regions.

Diamonds Colored by NV0/– Centers. Diamonds colored by NV0/– centers (i.e., those with NV0/– centers in sufficient concentrations to be detected by Vis-NIR absorption spectroscopy) will have high-intensity NV0/– peaks in their PL spectra (figure 16). Due to this high intensity of the NV0/– centers and their associated vibronic structures, it can be difficult to detect other peaks that may be present at lower PL intensities. A peak at 561 nm is commonly seen, but the structure of this defect is still unknown. Common vacancy-related defects seen in diamonds with NV0/– centers include H3 (NVN0), H4 (4N-2V), and GR1 (V0).

GEMOLOGICAL OBSERVATIONS
Physical Characteristics. Pink and brown color is often associated with plastic deformation lamellae (“graining” or “slip/glide planes”). These are often observed as visible lines on the polished surface of the diamond, known as surface graining, which result from differential polishing rates along the deformation lamellae relative to the surrounding diamond. This leaves visible lines on the surface; unlike polishing features, these can cross multiple facets (figure 18, right).
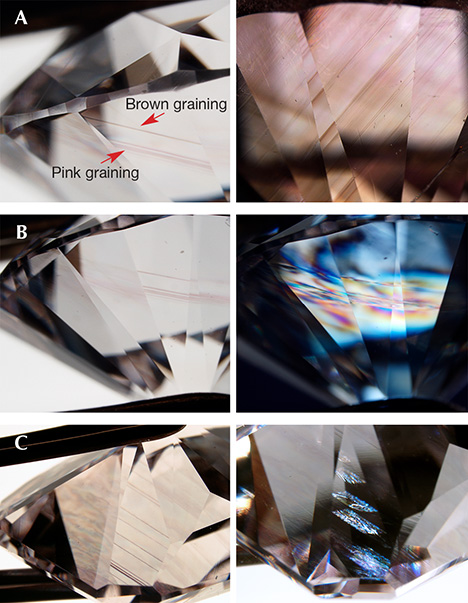

The graining usually has a hue similar to the diamond’s bodycolor, though in some cases diamond can exhibit both pink and brown graining (figure 17A). Additionally, graining and slip/glide planes can appear more pronounced when the diamond is put between crossed polarizers to show the strain (i.e., anomalous birefringence; figure 17B). The disruption in the strain pattern apparent at a slip/glide plane provides further evidence that these planes are a disruption of the diamond lattice (figure 17C).
Trigons can be etched where deformation lamellae intersect the surface of a rough diamond (figure 18, left). Where two of these deformation lamellae intersect, it is common to see an etch channel or thin needle-like structure (Wang et al., 2006). Diamonds are etched by fluids during their mantle residency or during kimberlite ascent (Fedortchouk et al., 2005; Fedortchouk and Zhang, 2011), and these fluids first exploit preexisting weaknesses in a diamond. Hollow channels known as Rose channels can also occur along intersecting twin lamellae (Schoor et al., 2016; figure 18, right). First described by Gustav Rose in 1868, these are formed by the intersection of twin lamellae, where the twinning action can result in a straight, hollow channel with a prismatic opening (Rose, 1868).
UV Fluorescence. 550 nm Band. Of the 992 “pink” diamonds colored by the 550 nm band that were randomly selected for detailed spectroscopic analyses, 786 had observations of long-wave (365 nm excitation) and short-wave UV (254 nm excitation) reported in the database. Among those with fluorescence observations, 571 (73%) showed blue fluorescence to long-wave excitation; 89 (11%) exhibited yellow fluorescence and 113 (14%) no fluorescence. Those with reported blue fluorescence generally showed medium (53%) to strong (25%) intensity and had a diamond type of IaA < B (70%). Those with reported yellow fluorescence to long-wave UV generally had very weak to weak intensity (88%) and were either saturated type Ia (42%) or IaA > B (36%). Those with fluorescence reported as none generally were either saturated type Ia (67%) or IaA > B (29%).
NV0/– Centers. The rare “Golconda pink” diamonds (six out of the subset of 1,000 samples, or 0.6%) all showed yellow-orange-red fluorescence colors caused by NV0/– centers. Although all colored diamonds should be submitted to a laboratory to determine the origin of their color, any “pink” diamond with yellow-orange-red fluorescence colors should be given special attention.
Bleaching. Many type IIa diamonds, including those colored by the 550 nm band and “Golconda pink” diamonds, change color after exposure to UV light. Although typically observed following exposure to the high-energy light of the DiamondView instrument (with wavelengths < 225 nm), bleaching can be observed following exposure to longer-wave UV but the effect is lessened at lower-energy, higher wavelengths (Fisher et al., 2009; Byrne et al., 2012b, 2014). After exposure to UV, “pink” diamonds can change color to yellow, brown, or near-colorless, and these changes are due to several charge-transfer processes that have been associated with the vacancy clusters present in brown diamonds (Byrne et al., 2014). Bleaching has also been observed during polishing of pink diamonds (Chapman, 2014).
The diamond’s color always reverts back to “pink,” though this can take anywhere from several minutes to more than a day depending on the color and intensity of the light (Byrne et al., 2014). Exposure to bright light (such as placing the diamond in a microscope light well) accelerates the process. As a precaution, GIA gemologists generally do not collect DiamondView images on “pink” diamonds unless other data indicate potential treatment, and DiamondView images are always collected after color grading. Any “pink” diamonds that require DiamondView imaging are returned to their stable color before leaving the laboratory.
Figure 19 shows the photos and spectra of a type IIa Fancy Intense pink diamond that was exposed to high-energy UV in the DiamondView. Its color dramatically and temporarily changed to Fancy Intense brownish yellow, which was accompanied by a decrease in the 390 nm and 550 nm absorption bands, while the intensities of the other centers (e.g., N3, H3, and GR1) remain unchanged.

IDENTIFICATION CONCERNS
Type Ia “Pinks.” There is no known method of treatment to create the 550 nm absorption band in the laboratory. Except for the rare type IaB diamonds discussed below, the majority of type Ia diamonds cannot be treated to appear pink. Additionally, the colored graining common in type Ia “pinks” cannot be artificially created.
Type IIa and Type IaB “Pinks.” As with the high-pressure, high-temperature (HPHT) decolorizing treatment, type IIa and type IaB pinkish brown diamonds can be HPHT-treated to remove the brown component (Hainschwang et al., 2003; Fisher et al., 2009). If the 550 nm band is present in these diamonds, the pink color will be accentuated by HPHT treatment, as the brown color is reduced. Diamonds that are HPHT-treated to pink are generally subjected to lower temperatures (1700–1800°C) than those that are HPHT-treated to colorless (2000–2300°C; Fisher et al., 2009; Dobrinets et al., 2013). As with colorless diamonds, these “pink” diamonds should be assessed by photoluminescence spectroscopy, which shows emission peaks that reliably distinguish between natural and treated diamonds. Additionally, a few “pink” CVD diamonds appear pink due to an absorption band at ~520 nm (Wang et al., 2007); therefore, careful assessment is needed to verify that the color-causing visible absorption band in type IIa “pink” diamonds is centered at ~550 nm. The cause of the ~520 nm band in CVDs is also unknown.
Type IIa “Golconda Pinks.” Any diamond colored by NV0/– centers must be examined extremely carefully. Both treated naturals and treated synthetics are altered to a pink color by generating NV0/– centers. Eaton-Magaña and Shigley (2016) provide a comparison table contrasting the distinguishing characteristics of natural, treated, and synthetic “pink” diamonds that are colored by NV0/– centers. Natural “Golconda pink” diamonds typically have pale colors, while treated “pink” diamonds have much more saturated colors. Although the separation of synthetic diamonds is generally less complicated than the separation of treated diamonds, a full complement of absorption, luminescence (such as mapping the spatial distribution of defects; D’Haenens-Johansson et al., 2017), and gemological data should always be collected and carefully evaluated.
UNUSUAL EXAMPLES
A small number of brown-red to red-brown diamonds owe their color to a combination of a slight 550 nm band with a 480 nm band (figure 20). The defect responsible for the ~480 nm absorption band also has a luminescence band centered at ~700 nm that is extremely broad with a unique wavy shape (figure 20, bottom). The combination of these two broad bands (the 480 and 550 nm absorption bands) leads to strong absorption from the orange to blue color regions. This combination of absorption features not only induced the common brown color but also created a “transparent window” in the red region. The result is a red-brown coloration.

CONCLUSIONS
Most colored diamonds receive the ingredients for color deep within the earth as they are created—nitrogen, boron, nickel, and hydrogen impurities produce yellow, yellowish green, violet, and blue colors, while abundant micro-inclusions can create white and black colors. After reaching the earth’s surface, diamonds can be exposed to radiation to create blue to green colors. However, the vast majority of natural “pink” diamonds colored by the 550 nm absorption band are produced through deformation processes deep within the earth.
Many source locality reports have been written about type Ia “pink” diamonds from Australia and Russia (Iakoubovskii and Adriaenssens, 2002; Titkov et al., 2008, 2012; Gaillou et al., 2010, 2012; Howell et al., 2015), yet very little has been written about type IIa “pinks,” and we could find no studies of a mine that regularly produces type IIa “pinks.” Therefore, one enduring question is the source locality of the type IIa “pink” diamonds colored by the 550 nm absorption band. Of the 992 diamonds colored by the 550 nm band that were randomly selected and studied in detail, almost one-fourth (243) were type IIa. Although this is a comparatively high percentage, little has been written about their source localities (e.g., Fisher et al., 2009; Smith et al., 2017).

Today, the Argyle mine produces a sizeable percentage of the world’s “pink” diamonds (e.g., figure 21), although pink to purple diamonds also come from countries such as Brazil, South Africa, and Russia. Since the Argyle mine is set to close within the next few years, the supply of “pink” diamonds, particularly those with saturated colors or those with red color descriptions, will decline unless the supply from other producers becomes more substantial.