Synthetic Emeralds Grown by Richard Nacken in the Mid-1920s
ABSTRACT
Chemical and microscopic examination of the first gem-quality synthetic emeralds of facetable size proves that Prof. Richard Nacken grew two main types of emerald by flux methods in the mid-1920s. One of these two types, grown with colorless beryl seeds in molybdenum-bearing and vanadium-free fluxes, has not previously been mentioned in the literature and would appear to be unknown to gemologists. The other main type, which has already been described in gemological publications, was grown from molybdenum- and vanadium-bearing fluxes. In drawing these conclusions, rough and faceted synthetic emeralds produced by Nacken were available for study from two principal sources: the Deutsches Museum in Munich, to which Nacken had donated samples in 1961, and family members who had inherited such crystals. Chemical, morphological, and microscopic properties are given, and circumstances concerning the developmental history of the Nacken production, including the possibility of collaboration with IG Farben (a subject of past speculation), are discussed as well. The latter has recently been elucidated by the discovery of original documents from the IG Farben gemstone plant, preserved in the Archives of the German Federal State of Saxony-Anhalt.
INTRODUCTION
The early technology for producing gem-quality synthetic emerald has long been a matter of speculation. In particular, the initial methods used for crystal growth by Interessen-Gemeinschaft Farbenindustrie Aktiengesellschaft, known as IG Farben, in Germany from 1935 to 1942 (“Synthetic beryl,” 1935; “Synthetischer Smaragd,” 1935), which yielded the so-called Igmerald, and by Carroll Chatham in the United States from 1941 onward have generated decades of discussion. Although detailed descriptions of both of these types of synthetic emeralds followed shortly after their introduction (for Igmerald see Eppler, 1935, 1936; Jaeger and Espig, 1935; Espig, 1935; Schiebold, 1935; Anderson, 1935; for Chatham synthetic emerald see Gübelin and Shipley, 1941; Anderson, 1941; Rogers and Sperisen, 1942; Switzer, 1946), the actual growth techniques remained unknown through the 1950s and were merely assumed to involve the hydrothermal method (Webster, 1952, 1955, 1958). Then, in the early 1960s, Hermann Espig, one of the inventors of the growth process used at IG Farben’s plant in Bitterfeld, near Leipzig, disclosed the technique as a flux-growth method using lithium-molybdate as the main flux component (Espig, 1960, 1961, 1962). Conversely, the precise composition of the flux used by Chatham has been a “closely guarded secret” (see Chatham, 1998). Nonetheless, the presence of traces of molybdenum in Chatham synthetic emeralds has established the use of a molybdenum-bearing flux for these stones as well (Nassau, 1976 a,b).
In gemological textbooks and overview articles summarizing the synthesis of gem-quality emerald (Cannawurf, 1964; Nassau, 1976 a,b; Schmetzer, 2002), the samples grown by Prof. Richard Nacken in Frankfurt, Germany, in the 1920s are frequently mentioned as early precursors of modern synthetic emeralds. Because Nacken did not publish any papers about the technique he used, and because only limited quantities of the resulting crystals went to museums or to teaching and research collections, many years passed before the growth method was proven through direct examination of samples. Rather, a hydrothermal technique was widely assumed, based on publications by Gordon Van Praagh (1946, 1947 a,b), whose work was also reviewed briefly in Gems & Gemology (Switzer, 1948) and therefore available to the gemological community. Van Praagh’s comments were derived from interviews with Nacken in 1945, covering primarily his research on hydrothermal synthesis of quartz (Nacken, 1950, 1953). The first detailed gemological study of Nacken synthetic emeralds, likely performed on samples loaned from the collection of Eduard Gübelin of Lucerne, Switzerland1, followed this assumption (Eppler, 1958 a,b). Only in the 1970s did examination by Kurt Nassau (1976 a,b, 1978) of Nacken synthetic emeralds, preserved in the collections of Frederick H. Pough of Reno, Nevada, and the Natural History Museum in London, identify residues of a molybdenum- and vanadium-based flux by scanning electron microscopy in combination with EDXRF analysis. This result was confirmed in Schmetzer et al. (1999), using samples from the collections of Pough and Gübelin1.
In seeking to place Nacken’s work in the historical context of emerald synthesis, Nassau (1976 a,b, 1978) mentioned some similarities with the crystal growth techniques used by Espig at IG Farben, and he speculated about a possible collaboration between the two scientists. Nassau also suggested that the secret coloring ingredient in Igmeralds alluded to by Espig (1960) might have been vanadium, but this uncertainty has since been resolved by identification of a nickel-bearing compound used in addition to chromium to achieve the desired color (Schmetzer and Kiefert, 1998). In contrast, the question about collaboration between Nacken and the IG Farben researchers at Bitterfeld has remained open. Moreover, it should be realized that the few known gemological and analytical examinations were based on samples obtained from a limited number of reliable private or public collections. Material sourced directly from Nacken has not been examined to date, simply because his synthetic emeralds have never been available commercially and only a few samples were donated to colleagues. Consequently, the possibility persists that other types of synthetic emeralds, grown by a variant of the flux method or by another technique, might have been produced. The present paper endeavors to incorporate new details into the decades-old discussion on the history of synthetic emerald.
PROF. RICHARD NACKEN (1884–1971)
Richard Nacken2 was born May 4, 1884, in Rheydt, near Mönchengladbach, Germany, and died April 8, 1971, in Oberstdorf. From 1903 he studied mathematics and natural sciences at the Universities of Tübingen and Göttingen (figure 1), graduating from the latter in 1907. He worked as an assistant at the University of Göttingen until 1908 and at the University of Berlin from 1908 to 1911. In 1911, at the age of 26, he was appointed associate professor for mineralogy and petrology at the University of Leipzig, making him the youngest mineralogy professor in Germany at that time. In Leipzig he also met his wife Berta (née Dreibrodt), and the couple married in August 1912. Nacken’s academic career continued with positions at the Universities of Tübingen as associate professor (1914–1918), Greifswald as full professor (1918–1921), and Frankfurt as director of the mineralogical institute. From 1936 he also served as director of the Institute for Gemstone Research at Idar-Oberstein3. After the mineralogical institute at the University of Frankfurt was completely destroyed in the spring of 1944 by Allied bombing (and his home was likewise heavily damaged), Nacken moved to the countryside near Schramberg in the Black Forest, where he was able to set up a small laboratory at the Junghans Watch Company4. In 1946 he returned to the University of Tübingen as professor, a position from which he retired in 1952.
Nacken’s primary academic interest was in crystal growth and mineral synthesis techniques. Particularly notable among his work were two discoveries. One was the invention of a method for crystal growth in which a seed was attached to a cooled copper rod and inserted into a melt, thereby instigating growth on the cooled seed (Nacken, 1915, 1916). This method is still known as the Nacken-Kyropoulos technique (based upon a variant developed by Spyro Kyropoulos in the mid-1920s; see Feigelson, 2014). A second significant contribution was Nacken’s advancement of the technology for growing quartz hydrothermally in autoclaves, based mainly on his research from the late 1930s until 1945 but published in the 1950s (Nacken, 1950, 1953). His hydrothermal work was of interest to foreign governments in the years following World War II and was covered in five documents prepared under the auspices of American and British government entities. These included the U.S. Army’s Field Information Agency, Technical (FIAT) and the British Intelligence Objectives Sub-Committee (BIOS); relevant documents are archived as PB 6498 (Guellich et al., 1945), PB 14620 (Sawyer, 1945), PB 18784 (Swinnerton, 1945), PB 28897 (Swinnerton, 1946), and BIOS Final Report No. 552 (Coates, undated). These documents were frequently cited in publications addressing subsequent developments in hydrothermal quartz synthesis for crystal oscillators between 1945 and 1960. Some of these documents also contain limited information about emerald synthesis5, but Nacken never described his method in a scientific paper. Circumstances that might have motivated Nacken’s efforts in growing emeralds will be discussed below.
In addition to the achievements above, Nacken authored numerous papers in the 1930s dealing with phase diagrams and formation of cement minerals, research that had begun as early as 1919. Other work in the gemological field included contributions to the Verneuil technique for growing rubies and sapphires (German and British patent documents, published 1925 and 1926) and a method for distinguishing between natural and cultured pearls (German, British, French, Swiss, and Austrian patents, published in 1927 and 1928).
MATERIALS AND METHODS
In October 1961, Nacken donated a glass vial to the Deutsches Museum (German Museum) in Munich, Germany’s largest museum for science and technology. The closed ampoule, inventory number 75218 (figure 2), was described at the time as containing synthetic emeralds grown from 1923 to 1925. This information about the growth period is, to the knowledge of the present authors, the only firsthand information available directly from Nacken. The glass ampoule remained closed until being opened for the first time for this study. Inside were 360 faceted synthetic emeralds and 10 crystals or crystal fragments. The total weight of the samples was 11.15 carats.
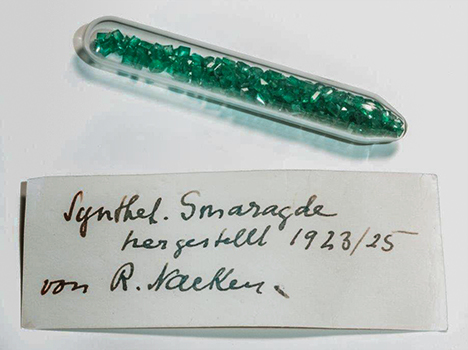
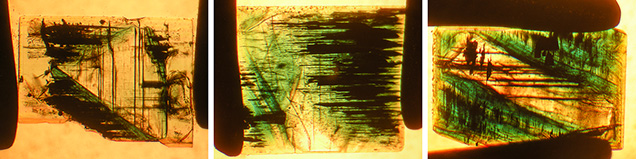
During the course of our investigation, we also learned that a small mineral collection left by Nacken at his death has remained within the family and is in the possession of his grandsons. Contained in a wooden box, this collection similarly comprised various glass ampoules with synthetic gem materials, primarily ruby, sapphire, and spinel boules grown by the Verneuil method. Two such glass containers held synthetic emeralds, but the labels (figure 3) indicated no further details. From these two containers, 77 faceted synthetic emeralds and rough crystals, weighing a total of 9.73 carats, were kindly loaned by E. Schlatter, Nacken’s grandson, for this research project. These samples (figure 4) covered all different color varieties and sizes within the two lots. This material had never been examined by mineralogical or gemological methods and had only been inspected visually by family members. No written documentation pertaining to these synthetic emeralds or any other technical information regarding Nacken’s work was available from the family.


For comparison, we also examined seven rough samples from the collection of one of the authors (KS). These were initially loaned by Drs. Gübelin and Pough to the author for study and donated after the related paper (Schmetzer et al., 1999) was published. Another synthetic emerald crystal was obtained for comparison from the reference collection of the German Gemmological Association in Idar-Oberstein.
All 77 samples submitted by the Nacken family (rough and faceted) and approximately 150 faceted synthetic emeralds as well as the 10 rough samples from the vial donated to the Deutsches Museum were examined microscopically in immersion. Visually, it soon became apparent that two primary groups seemed to exist. From these two groups, a total of 75 rough and faceted synthetic emeralds, again covering all sizes and color varieties from the Deutsches Museum and Nacken family samples, were selected for X-ray fluorescence spectroscopy (EDXRF) using a Bruker Tracer III-SD mobile unit. These samples were also examined at higher magnification (up to 1000×) with a Leica DM LM polarizing microscope employing a transmitted light source. For documentation, we used an Olympus DP25 digital camera with Olympus Stream Motion 1.6.1 software.
The previously mentioned eight samples available for comparison from various collections, which were more indirectly linked to Nacken, were similarly examined by the EDXRF and optical methods described in the preceding paragraph.
RESULTS
Chemical, mineralogical, and gemological properties of the Nacken synthetic emeralds studied are summarized in table 1 and described more fully below.
Chemical Properties. The trace elements revealed by EDXRF clearly distinguished two principal groups of synthetic emeralds (figures 4 and 5), referred to as type 1 and type 2 in the present study. Type 2 comprised samples grown from a molybdenum- and vanadium-bearing flux, and stones of this type have previously been documented and described (Nassau, 1976 a,b, 1978; Schmetzer et al., 1999). Conversely, the type 1 samples were grown from a molybdenum-bearing flux without vanadium, as the latter element was absent from their X-ray spectra. This type of Nacken synthetic emerald has, to the best of our knowledge, never been mentioned in the literature. Chromium was the main color-causing trace element in both types, with small traces of iron also present.
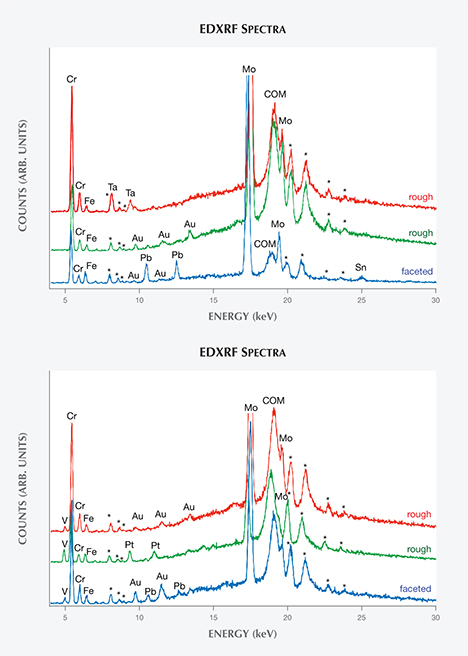
A third small group, consisting of two samples from the Pough collection, was also grown from molybdenum- and vanadium-bearing fluxes. However, because these two samples differed from the main type 2 group in a key microscopic feature—the absence of a colorless seed—they were separated and designated type 3. No type 3 stones were discovered within the samples from the Deutsches Museum or the Nacken family.
The variable intensities of the chromium, vanadium, and molybdenum peaks in the EDXRF patterns indicated that Nacken experimented with various mixtures of fluxes and color-causing trace elements in an effort to obtain the ideal conditions for crystal growth and color. In a few samples, in which large portions of the natural colorless seed (see below) were exposed to the surface, signals assigned to traces of gallium were also observed.
Characteristic X-ray lines for gold or tantalum (in type 1 samples) or for gold or platinum (in type 2 samples) were frequently seen. These emission lines could not be explained by residual flux and were therefore assigned to particles from the crucibles or other containers used for emerald synthesis. In both types, traces of gold were observed most often. Only a smaller fraction of type 1 samples showed tantalum lines, while a similarly small fraction of type 2 samples showed platinum lines. In faceted samples of both types—but not in rough crystals—the characteristic X-ray emission lines for lead or for lead and tin were occasionally present. These trace elements were artifacts of the polishing process, which was performed at that time on wheels composed of lead or a lead-tin alloy.
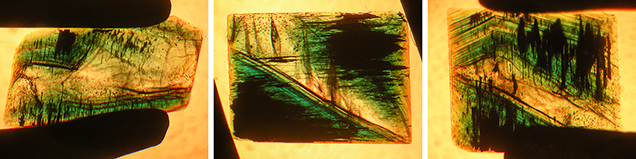
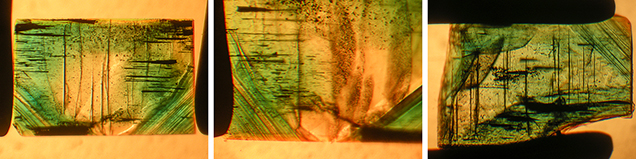
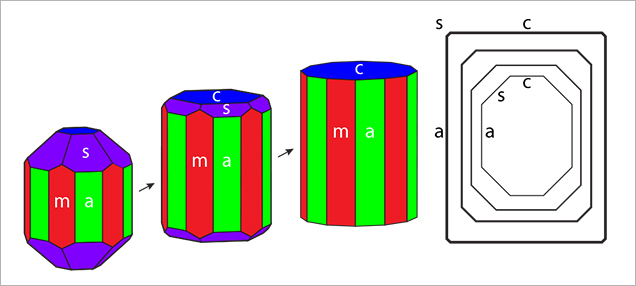
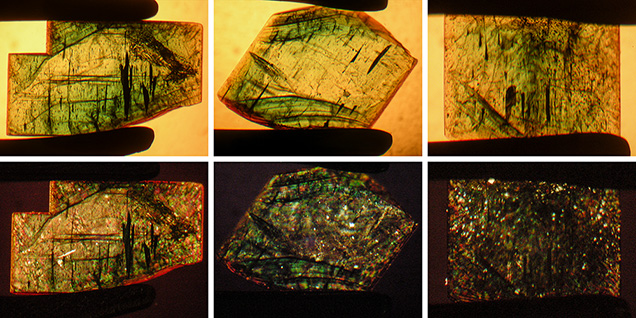
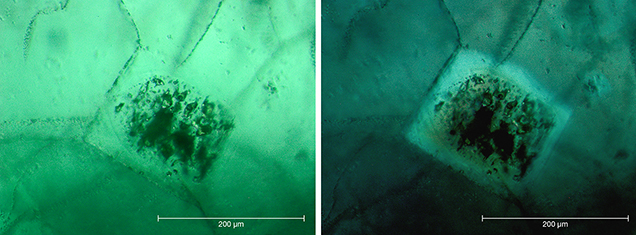
Crystal Morphology. The rough samples of all three types were prismatic crystals with a habit formed by first- and second-order hexagonal prism faces m {1010} and a {1120}, in combination with the basal pinacoid c {0001} (figure 6). Due to the external form of the natural seed used for growth, the majority of the synthetic emerald crystals were moderately distorted, and in most cases only a subset of the faces of the twelve-sided prism could be observed, normally between eight and ten. An idealized (undistorted) crystal with all twelve prism faces is shown in figure 6 (left and right insets). Some of the crystals, especially from type 1, exhibited somewhat rough and uneven faces and rounded edges, possibly indicating minor dissolution of the completed crystals at the end of the growth period. The largest rough crystal measured approximately 8 × 6 × 5 mm, with a colorless seed of about 6 × 4 × 3 mm, and weighed 1.32 ct. The largest faceted synthetic emeralds approached 4.5 mm in one dimension for rectangular stones (e.g., 4.5 × 3.0 mm, 4.2 × 2.3 mm, or 4.0 × 2.5 mm) and weighed up to 0.15 ct, but most were between 0.05 and 0.10 ct.
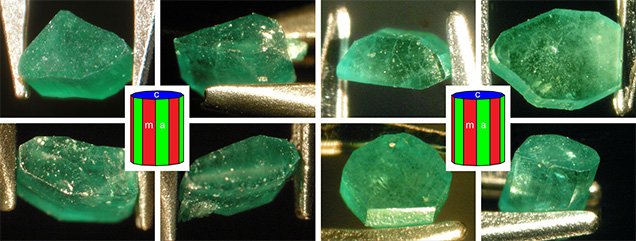
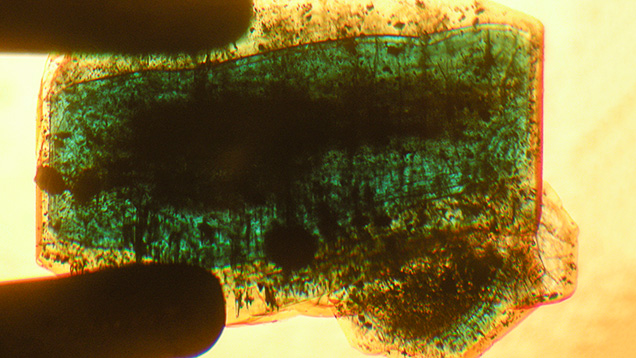
Microscopic Features. Colorless seeds, mostly in the 1–2 mm range and rarely as large as 5 mm, were found in all samples obtained from the Deutsches Museum and the Nacken family, as well as in most of the samples available from other collections for comparison (figures 7 and 8). Certain of these seeds, sometimes not larger than 0.5 mm, were not easily recognizable, especially in larger and heavily included stones. Nonetheless, careful examination with the immersion microscope revealed the presence of such irregularly shaped colorless seeds in all the synthetic emeralds with the exception of two heavily included samples obtained by one of the authors (KS) in the late 1990s from the Pough collection. As mentioned above, these samples were separated from the two main groups of synthetic emeralds and designated as type 3 (see table 1).
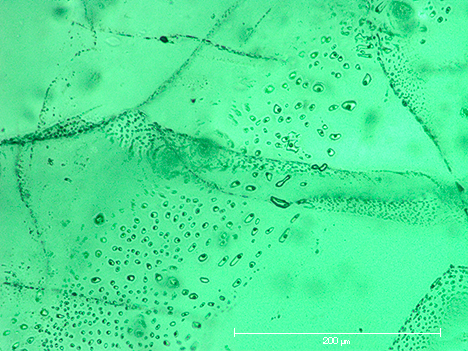
The colorless seeds frequently showed fluid inclusions on planes parallel to the basal pinacoid of the seed crystals (figures 7 and 8). Natural emeralds with such an inclusion scene are typical for some locations, as observed in samples from the famous Russian deposits in the Ural Mountains (Schmetzer et al., 1991).
The colorless beryl seeds showed no attachment or suspension points. The absence of such features indicated that the seeds floated freely within the molybdate or molybdate-vanadate melt. Even clusters of synthetic emeralds, consisting of three or four intergrown prismatic crystals, contained small seeds in each of the tiny crystals (figure 9). This could be established by careful inspection of type 1 and type 2 clusters, guided by the trace-element pattern generated through EDXRF.
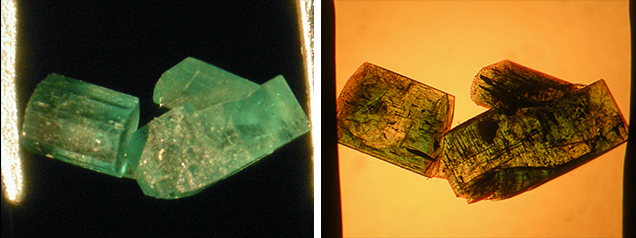
In the two type 3 samples from Pough examined for comparison, no colorless seeds could be detected. Both crystals were grown in a molybdenum- and vanadium-bearing flux and were heavily included. They also showed the typical morphology of Nacken synthetic emeralds. Although not as clearly outlined as the colorless seeds in the other samples, both stones displayed a somewhat deeper green core (figure 10), which could suggest the presence of a natural or synthetic emerald seed.
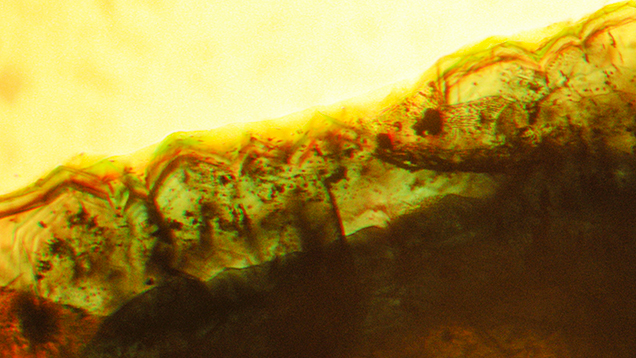
In type 1 and type 2 synthetic emeralds, growth layers in the first stages of crystal growth followed the external shape of the seed (figures 11 and 12). Subsequent growth proceeded through a period dominated by the hexagonal dipyramid s {1122}, inclined 45° to the c-axis of the emerald crystals (figure 12). This face initially developed more rapidly than the prism faces m and a and the basal pinacoid c. In still later stages, the s face therefore became obscured and was no longer apparent on the surface of the final rough crystals with prismatic habit (figure 13).
It is known that synthetic emerald starts to dissolve in a molybdate melt if the supersaturation of the various components is not sufficient, especially at the end of a growth cycle (Espig, 1960, 1961, 1962). Some irregular growth features in the form of irregular zigzag patterns were observed in several of the crystals (figure 14). These oscillating growth structures were possibly caused by unstable growth conditions, which could include such dissolution processes.
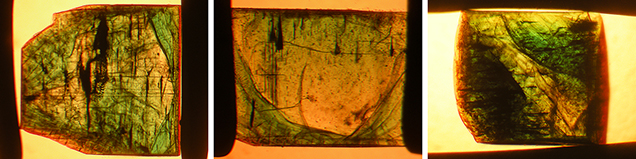
Color zoning in type 1 samples was generally strong, and the following variations could be distinguished:
- Very intense green growth layers fading gradually from the center outwards, becoming lighter green or almost colorless in the outer regions. In some samples, this pattern with gradually decreasing color intensity repeated several times, indicating multiple growth cycles for the synthetic emerald crystals (figure 15).
- Alternating intense green and lighter green to almost colorless growth layers, but with sharp boundaries between the intensely colored and lighter areas (figures 11, right, and 12, left and center).
- Intense green growth (incorporating a small colorless seed) with a light green or almost colorless overgrowth, seen in only a few samples (figure 16).
In type 2 samples, only weak color zoning with alternating lighter and darker green zones was observed (figures 8 and 12, right).
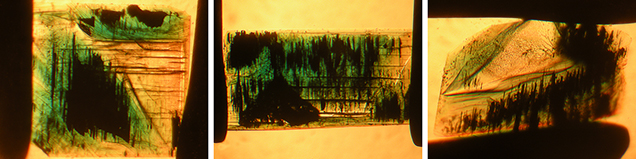
Type 1 samples in general were heavily included (figures 7 and 15). The most characteristic features were growth tubes filled with residual transparent or non-transparent flux (figure 17, A–D). These tubes ran parallel to the c-axis and were often conical in shape. When the residual flux was transparent, a contraction bubble was typically visible. Occasionally, a small birefringent crystal was attached to the wider end of the growth tubes (figure 17E). Such crystals were most often found close to the boundary between the natural colorless beryl seed and the green emerald overgrowth. The tiny crystals were birefringent but had a refractive index similar to that of the host. Consequently, they were clearly visible only under crossed polarizers. Wispy veils of residual flux were also common in type 1 synthetics (figure 17F).
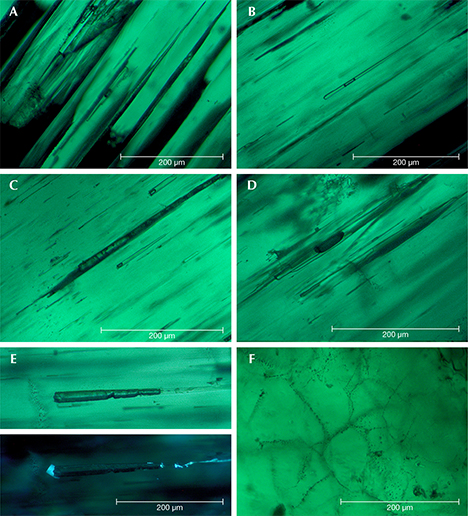
In general, type 2 samples were less heavily included (figure 8). Conical growth tubes analogous to those seen in type 1 samples were present, but the tiny birefringent crystals attached to the wider ends of the growth tubes were more common. Again, the similarity in refractive index with the host meant that these crystals were best revealed under crossed polarizers, as were other tiny inclusions of the same type not attached to growth tubes (figure 18). The birefringent crystals at the ends of the tubes also frequently contained small inclusions (figure 19, A–E). The residual flux within the growth tubes ranged from transparent with a contraction bubble to inhomogeneous, non-transparent, and even birefringent (figure 19, A–E). Isolated tubes without birefringent crystals at the wider ends were rare in type 2 stones (figure 19F). Somewhat irregularly shaped inclusions with various forms of residual flux—transparent or non-transparent, birefringent or non-birefringent—were also seen occasionally (figure 20). Additionally, small particles of residual flux trapped in veil-like feathers were found, often with contraction bubbles visible at higher magnification (figure 21).
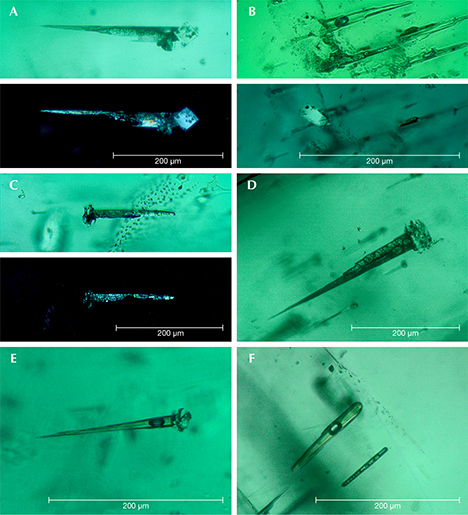
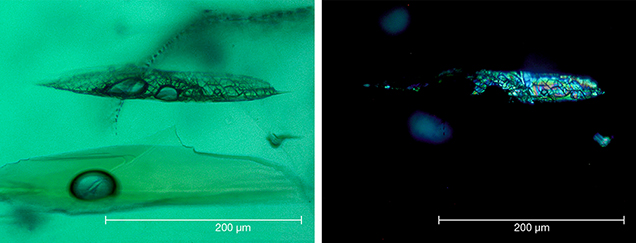
The inclusion pattern consisting of conically shaped growth tubes capped by birefringent crystals, forming what gemologists refer to as nail-head spicules, has been the subject of several prior works on Nacken synthetic emeralds. An early description was provided by Wilhelm F. Eppler (1958 a,b). The birefringent crystals were later examined in detail by Raman micro-spectroscopy and identified as tiny emerald crystals with an orientation different from that of the host (Schmetzer et al., 1999). The substances found in the growth tubes were likewise further characterized using micro-chemical and micro-spectroscopic techniques such as electron microscopy, electron microprobe analysis, and Raman micro-spectroscopy (Nassau, 1976 a,b, 1978; Schmetzer et al., 1999). They consisted of mixtures of the flux components (vanadium and molybdenum) and the synthetic emerald ingredients (aluminum, silicon, and chromium). These fillings were either transparent, representing a glassy state, or translucent to opaque, and sometimes even partially birefringent, representing multiple states of phase separation (e.g., formation of a bubble in the melt), as well as incomplete and complex devitrification processes including recrystallization of the flux after cooling. Interestingly, various forms of trapped flux in different states were found close together in the same host crystal.
Type 1 and type 2 synthetics also occasionally showed included emerald crystals not attached to growth tubes. Such inclusions had been formed by spontaneous nucleation. Larger inclusions of this nature contained various forms of residual flux (figure 22), comparable to the inclusions seen in the synthetic emerald host.
Tiny opaque platelets with a hexagonal or trigonal shape were another notable inclusion feature (figure 23). Analogous platelets have been recognized in flux-grown gem materials (e.g., emeralds, rubies, or sapphires) and identified as platinum platelets originating from the platinum crucibles used for crystal growth. In the present study, trace-element analyses most often indicated a gold component corresponding to growth in gold containers. Because hexagonally or trigonally shaped gold platelets are known to be formed under various synthetic growth conditions (Morriss et al., 1968; Smart et al., 1972; Engelbrecht and Snyman, 1983; Lofton and Sigmund, 2005; Liu et al., 2006), characterizing the platelets evident in many Nacken emeralds as gold seems reasonable. Similar gold platelets with hexagonal and trigonal outlines were described in Biron synthetic emeralds grown in Perth, Australia (Kane and Liddicoat, 1985).
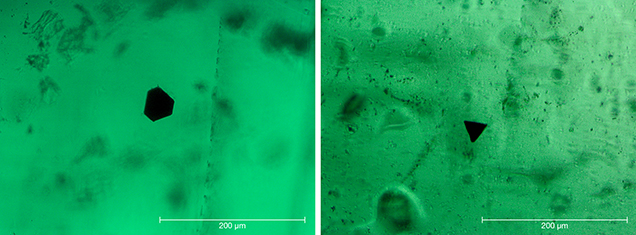
Type 3 Nacken synthetic emeralds exhibited a similar inclusion pattern of nail-head spicules, tiny isolated beryl crystals, and various forms of flux.
TECHNOLOGY OF CRYSTAL GROWTH
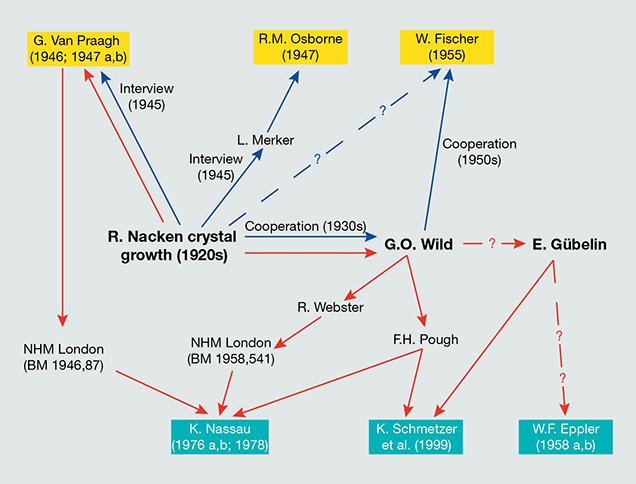
A general overview tracking the primary information published to date about the crystal growth technology applied by Nacken in the 1920s (Van Praagh, 1946, 1947 a,b; Osborne, 1947; Fischer, 1955) and the sources for the research samples underlying the major extant gemological descriptions of these synthetic emeralds (Eppler, 1958 a,b; Nassau, 1976 a,b, 1978; Schmetzer et al., 1999) is given in figure 24. All samples previously examined were grown in molybdenum- and vanadium-bearing fluxes. In contrast, the samples submitted by Nacken to the Deutsches Museum consisted mainly of faceted stones grown without vanadium as a component of the flux. Another sample grown in a vanadium-free flux was found in the reference collection of the German Gemmological Association during the course of this study. The samples loaned to the authors by the Nacken family comprised both principal types.
Primary Sources. As previously noted, various Allied government documents were generated after World War II from investigations into German scientific activities. In several of these, dealing mainly with growth of quartz and other piezoelectric crystals, isolated statements by Nacken about synthetic emeralds can be found5, but only PB 85147 contains significant information. Written by R.M. Osborne (1947) and based on an interview of Nacken by Leon Merker6, this report notes:
A stoichiometric mixture of alumina and beryllia is prepared as a paste and dried. This is gradually fed into a molten mixture of potassium carbonate and vanadium pentoxide at 900–950°C in a gold crucible. Quartz is simultaneously fed into the melt in the form of small quartz pieces. The crystallization process is seeded with small emeralds and beryls. The crystallization is slow and only small emeralds are said to be produced. It was stated that the emeralds could be increased in size by removing them from the melt in which they were grown and placing them in a fresh melt of the same composition.
In contrast to this clear description of a flux-growth process, Van Praagh7 (1946, 1947 a,b) described Nacken’s synthesis of both emeralds and quartz as hydrothermal. He stated that Nacken synthesized different minerals “by crystallizing them from water or dilute aqueous solution in the neighborhood of the critical temperature of water. By 1928 he had succeeded in synthesizing a number of minerals including feldspars, mica and beryl.” Van Praagh further mentioned growth on a seed crystal in an autoclave:
The vessel of the autoclave was lined with silver and the seed crystal was suspended from a silver wire. The rough material consisting of a mixture of beryllium oxide, alumina and silica in the correct proportions was placed in the autoclave, which was then closed and the temperature raised to about 370–400°C. It was maintained at this value for a few days…. Finally Nacken was able to make crystals of beryl (colored green with a trace of chromium to turn them into emeralds) that were up to 1 cm in length and 2 to 3 mm in width.
In 1955, Walther Fischer8 wrote a paragraph about Nacken’s synthetic emeralds in an encyclopedia review article about synthetic gemstones [translated from German]:
In 1925 R. Nacken succeeded in growing emerald crystals of remarkable size. He suspended a beryl seed crystal within a melt consisting of acid lithium molybdate to which he added a mixture of Al2O3, SiO2, and BeO. In addition to the presence of small amounts of water, an excess of SiO2 is important, which has to be calculated according to the other components. To achieve the desired coloration, traces of Cr2O3 and Fe2O3 are added. The application of pressure is unnecessary. Great difficulties came from problems with the appropriate selection of the right materials. Nacken worked with gold and silver containers.
Correlation of Primary Sources with Experimental Results. The results of the present study are consistent with previous research (Nassau 1976 a,b, 1978; Schmetzer et al., 1999), insofar as they confirm flux growth of Nacken synthetic emeralds. The experimental results also augment the existing literature through identification of a new type of Nacken synthetic emerald. In addition to those stones known to have been grown from molybdate-vanadate fluxes (designated as types 2 and 3 herein), a variation grown from molybdenum-bearing fluxes without a vanadium component has now been identified. Conversely, we discovered no Nacken samples lacking entirely in flux residues (as proven by a combination of EDXRF and microscopy), which would have suggested hydrothermal growth. This conspicuous absence raises questions about the origin of the information detailed in Van Praagh’s publications (1946, 1947 a,b).
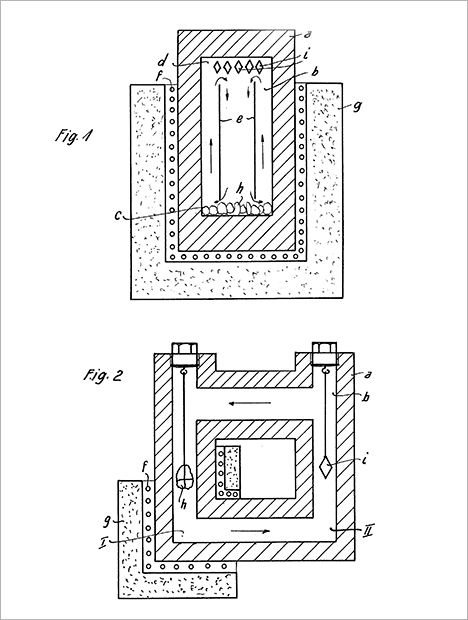
As noted above, Van Praagh and other American and British government personnel interviewed Nacken several times in 1945 to acquire scientific and technical information about the German program for substituting hydrothermally grown crystals for natural quartz in electronic devices9, as summarized by Swinnerton (1946) in document PB 28897. None of the government documents generated from these various interviews with Nacken mentioned any of his unpublished patent applications in this field. One such application10, originally filed June 25, 1943, by Nacken and Immanuel Franke, became German patent 913 649, published June 18, 1954.
The 1954 patent document described the use of autoclaves with vertical or horizontal temperature gradients for the growth of single crystals (figure 25). This technique became the basis for the development of a nascent industry, in the United States and elsewhere between 1945 and 1960, focused on growing quartz for technical applications such as crystal oscillators. That work, in turn, led to the modern-day mass production of synthetic quartz. Single-crystal quartz was highlighted as the most important material to be grown in the Nacken and Franke patent, but the patent also mentioned possible use of the method for synthesizing other minerals such as beryl, tourmaline, mica, fluorite, corundum, and asbestos. Whether those minerals had already been synthesized successfully was not indicated. Continued work by Nacken (figure 26) in the field of autoclaves and hydrothermal mineral synthesis is further evidenced by a patent document dated from the late 1950s (Nacken and Kinna, 1959).

According to the existing literature, hydrothermal synthesis of emerald began with Johann Lechleitner in Austria in the late 1950s or early 1960s (Holmes and Crowningshield, 1960; Schloßmacher, 1960; Gübelin, 1961; Schmetzer, 1991), and the method was only disclosed a decade later in several US patents assigned to the Linde Division of Union Carbide Corporation and in related scientific papers (Flanigen et al., 1967; Flanigen and Mumbach, 1971; Flanigen, 1971). Another development of the 1960s was the flux growth of synthetic emerald by spontaneous nucleation or by seeded growth with natural beryl or emerald used as nutrient (not starting from the separate chemical components of beryl). Successful results were achieved with a flux of lead vanadate (Linares et al., 1962; Lefever et al., 1962; Linares, 1967; Flanigen and Taylor, 1967; Koivula and Keller, 1985; Bukin, 1993). Commercial growth of gem-quality synthetic emerald using natural beryl as nutrient has been documented for flux material produced by Gilson near Calais, France (Diehl, 1977), and for the Biron hydrothermal synthetic emerald produced in Perth, Australia (Brown, 1997).
Given the foregoing circumstances, the logical conclusion is that misunderstandings between Nacken and Van Praagh during the 1945 interview led the interviewer to assume that the synthetic emeralds grown in the 1920s had been created hydrothermally. Yet nothing suggests that the technology for hydrothermal growth of emerald was available in the 1920s. Furthermore, Nacken’s application of such a technique cannot be proven by any known samples and would seem particularly unlikely since his primary work in developing the hydrothermal method for quartz growth occurred two decades later, between 1939 and 1945. Buttressing this conclusion, Van Praagh later donated samples he had obtained from Nacken to the Natural History Museum in London (Van Praagh, 1946, 1947a), and these were proven by Nassau (1976 a,b, 1978) to be flux-grown synthetic emeralds.
In contrast, the properties found in the present study of the flux-grown synthetic emeralds correlate with the data recorded by Osborne (1947, documenting Merker’s 1945 interview with Nacken) and Fischer (1955, using information given by Nacken or, more likely, his colleagues in Idar-Oberstein). Nonetheless, we must remember that the historical information is somewhat generalized in nature, given that it was obtained at least two decades after Nacken had stopped his practical work growing synthetic emerald and in a context where there would have been little incentive for him to disclose any more than basic background.
That said, the consistencies are notable. Both the crucibles and the fluxes indicated by the present study find corroboration in the Osborne and Fischer writings and in the practices of the time. EDXRF analysis proved that Nacken used gold containers for most of his synthesis experiments, and Osborne and Fischer likewise mentioned his use of gold crucibles. Platinum, found in a few samples as a trace element, was and is a common crucible material applied in various chemical technologies, while tantalum is a cheaper alternative to platinum and also offers high corrosion resistance (Kieffer and Braun, 1963). It is a matter of speculation whether Nacken seriously investigated the use of growth facilities larger or more complex than simple gold or platinum containers or crucibles, but it seems he at least tried to use cheaper materials such as tantalum. The two main components of the fluxes seen in this study, molybdenum and vanadium (in the form of a molybdenum-bearing compound for type 1 emeralds and a molybdenum-compound together with a vanadium-bearing compound for type 2), were similarly mentioned in the literature (lithium molybdate by Fischer, vanadium oxide by Osborne). Moreover, French scientists had long used both molybdenum and vanadium in fluxes to grow synthetic emerald, although not in combination, but the resulting crystals only reached about 1 mm in size (Hautefeuille and Perrey, 1888, 1890).
Another historical parallel is found in Osborne’s allusion to multiple growth cycles. From the present study, it can be concluded on the basis of color zoning that at least two, and occasionally three, growth cycles were performed for most of the samples, with a higher percentage of chromium incorporated into the beryl structure at the beginning of each growth cycle. A similar observation was made recently for flux-grown synthetic alexandrite (Schmetzer et al., 2012).
A final point of historical relevance derives from the prime technical challenge of the day. In the first half of the 20th century, emerald synthesis was plagued by the formation—through spontaneous nucleation—of clusters of tiny crystals. To avoid this scenario, IG Farben in Bitterfeld used platinum crucibles in which part of the nutrients (beryllium oxide and aluminum oxide) were separated from the remaining emerald component (SiO2 in the form of vitreous silica). As described by Espig (1960, 1961, 1962), this separation was accomplished by placing the BeO and Al2O3 nutrients at the bottom of the crucible, suspending beryl seed crystals below a platinum net or baffle in the upper part of the platinum container, and floating silica plates on top of the lithium molybdate melt (Schmetzer and Kiefert, 1998). Nacken, too, must have successfully applied the same principle of separating the nutrient into two parts (feeding a beryllia and alumina paste into the flux melt and separately adding quartz pieces), while also using beryl seeds (see Osborne, 1947).
POSSIBLE COOPERATION WITH IG FARBEN
In papers on the history of synthetic emerald (Nassau, 1976 a,b), and particularly in a detailed report on those grown by Nacken (Nassau, 1978), the possibility of cooperation between Nacken and Igmerald producer IG Farben was discussed. Despite differences identified in the fluxes they used, Nassau argued that Nacken’s lack of inclination to publish details about his emerald synthesis or to attempt commercialization might suggest a secret collaboration with the mineralogists at IG Farben in Bitterfeld, particularly Espig.
However, in his descriptions of the growth process applied at Bitterfeld for emerald synthesis (1960, 1961, 1962), Espig still followed the assumption prevalent in the 1950s literature and referred to Nacken’s work as hydrothermal. To have stated so in his 1962 publication indicates that Espig had no direct information about Nacken’s technology and, accordingly, that the two did not cooperate in developing crystal growth methods.
Nonetheless, historical sources establish communications dealing with such a possible collaboration in the mid-1930s. Specifically, a file containing correspondence between IG Farben and Nacken, along with related internal reports and comments by IG Farben staff members, was discovered by one of the authors during recent research into the industrial history of Verneuil synthesis at the Bitterfeld plant11 (Vaupel, 2015).
In 1933, Nacken contacted IG Farben and asked if there had been any progress in the emerald synthesis project. Later, in January 1936, Nacken consulted one of the company’s directors and offered to cooperate, mentioning that he had already grown synthetic emerald successfully. At that time, Nacken was likely aware of IG Farben’s breakthrough in emerald synthesis, announced in several mid-1935 articles, and he would also have been informed that the company did not intend to pursue commercial production (Jaeger and Espig, 1935). Nacken and IG Farben exchanged samples at the end of January 1936. Nacken remarked in an accompanying letter that while he had obtained growth of 2 mm per month, the optimal growth rate to produce clearer samples would be somewhat slower.
The three samples submitted by Nacken to IG Farben were inspected by Espig at the company and by E. Schiebold at the University of Leipzig. Schiebold (1935) had previously examined IG Farben samples and written about the properties of Igmeralds. Both prepared reports of their inspections. Schiebold specified that the samples were of synthetic origin, but neither report described whether the synthetic emeralds contained any natural or synthetic seeds. Each report also mentioned the presence of nail-head spicules. In contrast to his later publications from the 1960s (presumably influenced by Van Praagh’s writings), Espig concluded that the growth method might have been similar to that used by IG Farben. He added that, given the unfavorable bluish green color and high content of impurities, the quality of Nacken’s samples was no better, and possibly even worse, than that of the samples produced at Bitterfeld. Since the Igmeralds could be grown at rates between 1.2 and 3 mm per month, purchasing Nacken’s technical know-how offered no apparent opportunity for improving the quality or growth technology of the IG Farben production.
During a final consultation with Nacken and his lawyer in March 1936, IG Farben representatives stated that the question of whether the company would pursue commercial production of synthetic emeralds could not be answered affirmatively at that time. A decision to move forward would require an ability to produce larger samples of better quality. Therefore, they declined to pursue Nacken’s technology.
The facts described above, all based on recently discovered letters and internal IG Farben communications and reports11, prove that development of emerald synthesis by Nacken at the University of Frankfurt and by Espig at the IG Farben plant in Bitterfeld occurred independently. There was, however, another personal connection between Nacken and IG Farben that in all likelihood did play a role in the history of emerald synthesis.
In 1913 a young mineralogist named Otto Dreibrodt12 was hired by Elektrochemische Werke Bitterfeld (which became part of IG Farben in 1925). Prior to this employment, Dreibrodt had already invented an apparatus for crystal growth in solutions or melts, and in his new position he was tasked with developing novel techniques for crystal growth and applying known methods to new materials, including synthetic emeralds. Elektrochemische Werke Bitterfeld also applied for German and international patents covering the apparatus previously invented by Dreibrodt, after rights to the device had been transferred to the company by contract. A German patent 273 929 was granted in 1914, and the corresponding US patent was published in 192013.
To work with a melt and not only with hydrous solutions, the complex apparatus was constructed of platinum and used, at least temporarily, in Bitterfeld for emerald synthesis experiments14. However, problems with oversaturation of the melt and spontaneous nucleation of tiny emerald crystals or aggregates could not be solved, and no emeralds of facetable size were obtained from the experiments. Dreibrodt left the company at the end of 1926, three years before Espig’s major breakthrough in emerald growth at IG Farben (in the fall of 1929).
Nacken was one of Dreibrodt’s academic instructors at the University of Leipzig, and both scientists were focused on crystal growth and mineral synthesis. Moreover, after Nacken married Dreibrodt’s sister Berta in 1912, the two families were always in close contact (E. Schlatter, pers. comm., 2015). Nacken (1952) described and depicted Dreibrodt’s apparatus in a treatise on methods for crystal growth, and he presumably had become aware of the patent soon after its publication in 1914. In light of these many connections, it can be assumed that the two scientists discussed academic problems of mineral synthesis and that Nacken would have heard about IG Farben’s difficulties in developing a useful method for synthesis of larger, facetable emerald crystals. It is possible that such conversations prompted Nacken to contemplate alternative solutions, which, in turn, might have led to the development of his successful method for emerald synthesis.
It could be that Nacken contacted IG Farben in 1936 and offered his technique after noticing from the company’s announcements that commercial application of the Igmerald technology was not intended, despite almost two decades of research15. Nonetheless, it remains unclear why Nacken neither published his results nor tried to commercialize his technique in the mid-1920s. From a brief comment in a 1951 publication by Fritz Klein16, it appears that faceted synthetic emeralds produced by Nacken were shown to Klein for evaluation in the 1930s. In all likelihood, however, the reception given to Nacken’s synthetics by Klein or any other trade members queried would have paralleled the reaction of emerald dealers to the introduction of synthetic emeralds to the market by IG Farben. Stated succinctly, none of the German companies dealing in faceted natural emeralds were interested in supplementing their established product range of natural stones with synthetics. What is known is that Nacken never ceased to be interested in further developments in the field of emerald synthesis. Indicative of such ongoing curiosity, his grandsons found among his papers a newspaper clipping from 1965 that described the recent success in synthetic emerald growth achieved by Walter Zerfass of Idar-Oberstein.
CONCLUSIONS
During the mid-1920s, Richard Nacken was able to grow the first synthetic emeralds of facetable size and quality. New details about his work have emerged, augmenting previous studies that were based on a limited number of samples and interviews from the 1940s.
We have shown that Nacken produced synthetic emeralds of two principal types: (1) crystals grown in molybdenum-bearing fluxes, and (2) crystals grown in molybdenum- and vanadium-bearing fluxes. Both were created primarily in gold containers using a process that added various ingredients such as BeO and Al2O3 separately from the third major beryl component, SiO2. Nacken typically employed small, colorless, irregularly shaped beryl seed crystals of 0.5 to 5 mm and attained faceted synthetic emeralds reaching up to 4.5 mm and 0.15 ct. Characteristic internal features include colorless cores, color zoning, and oriented inclusions such as nail-head spicules, all of which reflect the experimental conditions of crystal growth. The two principal types of synthetic emeralds are distinguishable by differences in growth and color zoning and inclusion features, in combination with chemical properties.
From a historical perspective, it has also become clear that Nacken developed his synthesis method independently, apart from any association with IG Farben’s Igmerald growth technique. Contemporaneous files establish that although Nacken discussed possible collaboration with scientists at IG Farben in the mid-1930s, no joint efforts were undertaken.
NOTES
- Wilhelm F. Eppler (1958 a,b) described three types of synthetic emeralds: samples grown by Nacken, Igmeralds produced by IG Farben in Bitterfeld, and Chatham synthetic emeralds. In giving credit for loan of samples, he acknowledged Eduard Gübelin, Hermann Espig, and Basil W. Anderson.
Eppler had been associated with Nacken in 1937, publishing papers through the Institute for Gemstone Research while Nacken was formally the organization’s director (Eppler, 1937 a,b; see note 3 and accompanying text). In a later publication, Eppler (1964) still assumed that Nacken synthetic emeralds were grown hydrothermally. It would thus seem that Eppler never received any direct information from Nacken about his growth technique.
The three lots examined by Kurt Nassau were loaned by Frederick H. Pough and by the Natural History Museum in London. According to the records of the Natural History Museum (M. Rumsey, pers. comm., 2015), the museum received two groups of synthetic emeralds, one donated by Gordon Van Praagh in 1946 (see note 7) and another donated by Robert Webster in 1958 (identifying Georg Otto Wild as the previous owner; see note 3). A letter from Webster to Wild, dated April 1948, acknowledged the gift of a Nacken synthetic emerald (D. Jerusalem, pers. comm., 2015).
The samples from the Pough collection loaned to one of the authors (KS) in the late 1990s had also been obtained from Wild (see note 3), but no further details were given. Pough spent two years (1931–1932) in Heidelberg, Germany, at the research institute of Victor Goldschmidt studying mineralogy and crystallography and thus would have had connections with German colleagues in the 1930s. In particular, there is evidence of a visit by Pough to Idar-Oberstein in mid-1937 that included a meeting with Wild (D. Jerusalem, pers. comm., 2015).
As for the samples traced to the Gübelin collection, a relevant detail is that Gübelin and Wild had known each other since 1937 (Gübelin, 1969).
In summary, all Nacken samples examined in the major papers written to date were sourced through three individuals—Van Praagh, Wild, or Gübelin—and samples in Gübelin’s collection may also have in turn come from Wild (see figure 24). - This paragraph is based on the most detailed published biographical data, as found in Kleber (1954) and Schloemer (1973); a draft of a personal autobiography by Nacken (circa 1959 or 1960); and on details provided by E. Schlatter, Nacken’s grandson, in 2015.
- From 1925 to 1936, the director of the Institute for Gemstone Research in Idar-Oberstein was Georg Otto Wild, who also founded the German Gemmological Association and became its first president in December 1933/January 1934. In the 1930s, Nacken was a board member of the association. When the German government decided to affiliate the Institute for Gemstone Research more closely with a university, Nacken (then with the University of Frankfurt) was named its director in November 1936, with Wild remaining the local leader for research activities and working in close collaboration with Nacken. Wild traveled to Frankfurt on a weekly basis during 1936 and 1937 and had an office in the university’s mineralogical institute (“Angliederung des Edelsteinforschungsinstituts Idar-Oberstein an die Universität Frankfurt a.M.,” 1936; Nacken, 1937; Chudoba, 1969; D. Jerusalem, pers. comm., 2015).
- Several interviews with Nacken were conducted by Allied investigators in 1945, when he was still living at Schramberg in the Black Forest (see note 5). Regarding the 1940s quartz synthesis program, the information about his work that he revealed to the interviewers would have been recent, insofar as he had pursued this topic at his small research laboratory in Schramberg following the destruction of the mineralogical institute at the University of Frankfurt. In contrast, the information given about his 1920s emerald synthesis would have been based solely on memory of a research project from two decades prior.
- In total, American and British teams conducted at least five interviews with Nacken in 1945 and possibly early 1946. The interviews were conducted by C.B. Sawyer (Guellich et al., 1945, archived as PB 6498; Sawyer, 1945, archived as PB 14620), A.C. Swinnerton (Swinnerton, 1945, archived as PB 18784; Swinnerton, 1946, archived as PB 28897), Gordon Van Praagh (mentioned in Swinnerton, 1946), F.H. Coates (Coates, undated, archived as BIOS Final Report No. 552), and Leon Merker (Osborne, 1947, archived as PB 85147). The various PB documents are preserved at the U.S. Library of Congress, Division of Science, Technology & Business, Washington, DC. In the interview by Sawyer it was only noted that Nacken displayed some synthetic emeralds from his own production. In Swinnerton’s interview, emerald was not mentioned. Coates observed that Nacken “appears to be more concerned with the production of synthetic emeralds…than pursuing the matter of synthesis of quartz.”
- Leon Merker (1917–2007), who studied chemistry at the University of Vienna and later in the United States at the University of Michigan, was one of the pioneers in applying the Verneuil flame-fusion method for growth of materials other than corundum and spinel, such as rutile, strontium titanate, calcium titanate, and aluminum titanate (all described in U.S. patents from the 1950s). Merker spent about one year at the U.S. Army’s Field Industrial Agency, Technical (FIAT), joining in 1945. During that period, he authored a report about the German synthetic stone industry (Merker, 1947). He mentioned that in 1945 he could not visit IG Farben’s Bitterfeld production plant, which was located in the Soviet-controlled zone of Germany. This fact supports a conclusion that the information about synthetic emerald given in document PB 85147 (Osborne, 1947) “by an old German scientist from memory” was based on a 1945 interview with Nacken. Subsequently, Nassau (1976 a,b, 1978) was able to communicate with Merker and to confirm that Nacken was the interviewee. Likewise, in a brief paper describing his own work on crystal growth technologies, Merker (2004) noted that he had the chance to interview Richard Nacken and Spyro Kyropoulos and that, back in the United States, he too had “toyed” with synthetic emeralds for a short time. Given his Austrian background, Merker would have experienced no language barrier in his communications with Nacken in 1945.
- Gordon Van Praagh (1909–2003) taught chemistry at Christ’s Hospital, in Horsham, Sussex, UK, and published several basic textbooks on chemistry and physical chemistry. He began working for the British Admiralty in 1943 during World War II, and his duties immediately after the war involved Allied combined intelligence operations, tracking down German scientific developments and expertise (Berry, 2003). In his articles about emerald and quartz synthesis, Van Praagh (1946, 1947 a,b) did not identify any sources for the information given; however, his interview with Nacken is mentioned in the American PB 28897 report (Swinnerton, 1946). Furthermore, in a later paper on quartz synthesis (1949), he referenced “Nacken (private communications, 1945)”.
- Walther Fischer (1897–1979) studied chemistry, mineralogy, and geology in Dresden, Germany, graduating in 1925. He left Dresden in 1948 and moved to Idar-Oberstein, where he worked until 1959 as a teacher and later served as the director of the local college for gemstone cutting and processing. His list of scientific publications (Metz, 1972) contains 380 titles, including many book reviews and historical articles. In his 1955 review article on synthetic gemstones, Fischer offered numerous references for most of the information given. In contrast, only the section about Nacken is without citations. This suggests either that the author had direct contact with Nacken, or more likely, that he received his information from colleagues in Idar-Oberstein. Fischer had good connections with Georg Otto Wild—see the acknowledgements in Fischer’s 1954 book Praktische Edelsteinkunde (Practical Gemmology)—who in turn worked closely with Nacken in the 1930s (see note 3). Hence, the information presented in the 1955 article could have come through Wild.
- Until the end of World War II, the United States used exclusively natural quartz, primarily from Brazilian sources, for mass production of oscillator plates. Therefore, no American program for hydrothermal growth of single-crystal quartz existed from 1940 to 1945 (Thompson, 2007). Nacken’s research programs for growing synthetic quartz for oscillator plates, with financial support by the German government, are documented for 1939 and 1941 (Bundesarchiv Koblenz [Federal Archives], file nos. 13331 and 13332).
- The history of German patent 913 649 by Richard Nacken and Immanuel Franke reflects political circumstances between 1940 and 1950. Four patent applications related to hydrothermal growth of crystals were filed between July 1942 and August 1943 by Nacken and Franke, both of Frankfurt. After the arrival of American troops in Berlin in mid-1945, unpublished patent applications were documented on microfilms retained by the Allies, but by then the German patent office had ceased operation. Copies of these microfilms were submitted to the patent office after it reopened in Munich in October 1949, though only titles and short abstracts were published in Germany in the early 1950s. The full documents were released as part of a special project of the German Patent and Trade Mark Office beginning in 2014, and the four Nacken and Franke patent applications are now available under document numbers DE N 45 891 AZ, DE N 46 429 AZ, DE N 46 863 AZ, and DE N 46 979 AZ. Three of these applications dealt with methods for hydrothermal crystal growth, and the last of the series described an autoclave seal. After 1949 Nacken and Franke requested further examination of the most recent of the three applications concerning hydrothermal growth methods. This application, filed June 25, 1943, in turn covered most of the material set forth in the two earlier submissions filed in 1942 and 1943. The application was granted in 1954 and became German patent 913 649.
- Verneuil synthesis of ruby and sapphire commenced at Elektrochemische Werke Bitterfeld in 1910. In 1925, this company, including the plant for producing synthetic gem materials, was incorporated into the IG Farben group. Later, under Communist rule, the state-owned entity VEB Elektrochemisches Kombinat Bitterfeld took control of synthetic ruby, sapphire, and spinel production at the factory. Documents from all three periods are preserved in the Landesarchiv Sachsen-Anhalt, Merseburg (Archives of the German Federal State of Saxony-Anhalt, Department Merseburg) and at Kreismuseum Bitterfeld. The correspondence with Nacken and the IG Farben internal reports and notes related to Nacken synthetic emeralds are preserved at Merseburg, file number LASA, MER, I 506, No. 769.
- Otto Dreibrodt (1887–1941) studied mathematics and mineralogy at the Universities of Halle and Leipzig, graduating from the latter in 1912. One of his academic instructors at the University of Leipzig was Richard Nacken. Dreibrodt joined Elektrochemische Werke Bitterfeld in August 1913. Numerous German and international patents by Dreibrodt and assigned to Elektrochemische Werke Bitterfeld dealt with improvements to the Verneuil technique (e.g., for producing sapphires and spinels of various colors) and with chemical technology not related to the synthesis of gem materials. He left the company at the end of 1926. His personnel file still exists in the Landesarchiv Sachsen-Anhalt (see note 11), file numbers LASA, MER, I 506, No. 1068 and I 506, No. 1069, but the reason for his departure is unknown. Several Verneuil boules from his work remained with his daughter L. Dreibrodt, but no synthetic emerald samples are included (E. Schlatter, pers. comm., 2015).
- The U.S. patent for Otto Dreibrodt’s apparatus for crystal growth was apparently considered worthy of attention; it was reviewed briefly in The American Mineralogist (“An apparatus for growing large crystals,” 1921), not a common practice at that time.
- Landesarchiv Sachsen-Anhalt (see note 11), file number LASA, MER, I 506, No. 475.
- According to the records of the German Patent and Trade Mark Office (Patentrolle), Munich and Berlin, German patent 273 929 expired in February 1924 due to non-payment of annual fees, thereby ending any protection under the patent laws and any restriction on commercial use by others. Obviously, the company had lost interest in this technology.
- In a 1951 book chronicling his adventures in South America and especially with Colombian emeralds in the first decades of 20th century, Klein mentioned an episode in which 35 carats of faceted synthetic emeralds were submitted to him at a bank in Frankfurt for evaluation. In his book, Klein did not directly identify Nacken as the producer, but he described the samples as having been manufactured by means of an invention made parallel to that of the “great concern.” During that era, only two producers had grown synthetic emeralds of facetable quality and size in Germany, so the samples evaluated were either Nacken’s synthetics or Igmeralds made by IG Farben. Because Igmeralds were released to the public in 1935, the “great concern” was likely a reference to IG Farben, thus implying that Nacken emeralds were the subject of Klein’s investigations. Based on these circumstances, this episode would be dated to the 1930s, presumably the second half of the decade.



