Spinel Inclusion in Spinel

A 26.73 ct transparent dark pink-purple oval mixed cut (figure 1) was submitted to the Carlsbad laboratory for identification services. Standard gemological testing showed a refractive index of 1.719 and a hydrostatic specific gravity of 3.59. The stone fluoresced very weak red to long-wave UV light and was inert to short-wave UV. Observed using a handheld spectroscope, the stone showed thin absorption bands near 680 nm, with fine absorption lines near 670 nm. These gemological properties were consistent with spinel.
Interestingly, microscopic examination showed that the spinel was relatively free of inclusions except for a very low-relief solid inclusion that was only partially visible (figure 2). Examination with polarized light (figure 3) revealed the entire outline of the low-relief crystal. Unaltered crystals generally provide strong evidence that a stone has not been heated, and this inclusion showed no alteration. But it is also important to consider the identity of certain inclusions when using them as an indication for thermal treatment. Due to the low relief of this inclusion, it is very likely a spinel inside of spinel. Their matching refractive index explains why the inclusion is nearly invisible. Since the host and inclusion are the same material, we would not expect to see any alteration due to heat treatment. Therefore, the unaltered appearance of this inclusion cannot be used to determine that this spinel has not been heat treated.

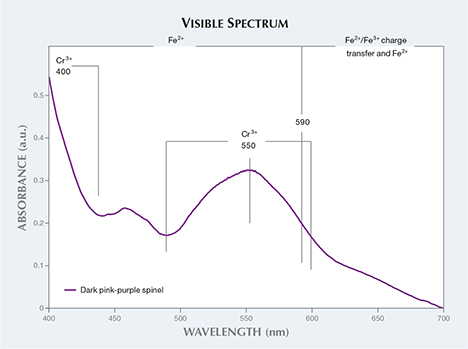
The spinel’s visible absorption spectrum revealed that its purple color results from the combination of Fe and Cr (figure 4). Absorption bands with a maximum at 400 and 550 nm are caused by Cr3+ (D.L. Wood et al., “Optical spectrum of Cr3+ ions in spinels,” The Journal of Chemical Physics, Vol. 48, No. 11, 1968, pp. 5255–5262). Fe2+ causes the absorption band from 400 to 590 nm, while the Fe2+/Fe3+ charge transfer and Fe2+ cause the absorption bands from 590 to 700 nm (S. Muhlmeister et al., “Flux-grown synthetic red and blue spinels from Russia,” Summer 1993 G&G, pp. 81–98).
Spinels submitted to GIA’s laboratory are routinely checked using advanced analytical tools. Laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) was used to confirm the natural trace elements. Higher concentrations of Li (27.7 ppmw), Be (87.4 ppmw), Zn (133 ppmw), and Ga (63 ppmw) indicate a natural origin, and the specimen’s photoluminescence (PL) spectrum was consistent with a heated spinel due to the broad full width at half maximum (FWHM) observed at 687.5 nm (figure 5; see S. Saeseaw et al., “Distinguishing heated spinels from unheated natural spinels and from synthetic spinels,” GIA Research News, April 2009, http://www.gia.edu/gia-news-research-NR32209A). It was unusual to see a spinel with this dark pink-purple color showing evidence of heat, as we typically see only red spinels that have been heated. From careful microscopic examination and advanced gemological testing, we were able to conclude that this was a natural spinel showing indications of heating.




