Unique Non-Bead Cultured Freshwater Pearls from Lake Biwa, Cultured for 14 Years
Lake Biwa in Shiga Prefecture has been producing pearls since 1928, when commercial freshwater pearl culturing first started in Japan. Although production decreased in the late twentieth century due to environmental issues and the declining mussel population (S. Akamatsu et al., “The current status of Chinese freshwater cultured pearls,” Summer 2001 G&G, pp. 97–113), Biwa pearls harvested in the twenty-first century, as well as vintage ones, are popular in the Japanese market. Hyriopsis schlegelii, an indigenous freshwater mollusk found in the lake prior to the 1980s, was originally used as the host for pearl culturing before it was replaced by the hybrid Hyriopsis schlegelii × Hyriopsis cumingii freshwater mollusk.

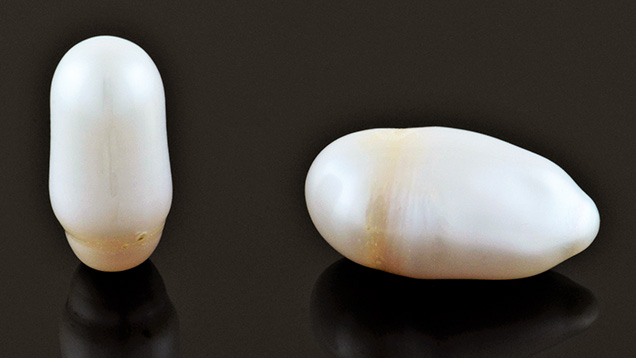
GIA’s Tokyo laboratory received for study 28 pearl samples with a stated Biwa provenance from Jinbo Pearls, a Shiga-based company that deals exclusively with freshwater pearls from Lake Biwa. Two of the largest samples (figure 1), weighing and measuring 16.08 ct, 19.75 × 10.38 × 9.84 mm (pearl A) and 22.85 ct, 23.48 × 12.36 × 11.08 mm (pearl B), respectively (figure 2, left), were selected for further detailed study. The intriguing fact was that they were reportedly grown over a period of 14 years—from 2002 to 2016. This is an unusually long growth period, since freshwater non-bead cultured (NBC) pearls normally take three years to form in Japanese farms. However, the farm where these samples originated was abandoned by the owner due to personal issues. Remarkably, several mussels were found alive when the farm was rechecked before being officially closed by the owner’s relatives in 2016. These two pearls were collected from two of the surviving mussels.

Real-time microradiography (RTX) examination revealed a faint linear feature along the length of each pearl (figure 2, center). X-ray computed microtomography (µ-CT) analysis showed these features more clearly and revealed an additional small dark void at the end of pearl A’s linear structure (figure 2, right). Their internal structures corresponded well to other non-bead cultured freshwater pearls described in previous studies (M.S. Krzemnicki et al., “X-ray computed microtomography: Distinguishing natural pearls from beaded and non-beaded cultured pearls,” Summer 2010 G&G, pp. 128–134). Additionally, optical X-ray fluorescence analysis showed a strong yellowish green reaction with weak yellowish orange areas within some imperfections around the circumference of the pearls (indicated by white arrows, figure 3, A-2 and B-2).
Each pearl was cut in half (cut surfaces were cleaned with isopropyl alcohol) and the examination results were compared with those obtained prior to sawing. Surprisingly, both showed stronger reddish orange optical X-ray fluorescence reactions within small areas along the central structure lines (RTX and μ-CT analysis) visible on each sawn face (figure 3, A-4 and B-4) than on any external surface areas when the pearls were intact. Such reactions are sometimes seen in natural and freshwater cultured pearls (Summer 2013 Lab Notes, pp. 113–114; Spring 2019 Lab Notes, pp. 94–96; S. Karampelas et al., “Chemical characteristics of freshwater and saltwater natural and cultured pearls from different bivalves,” Minerals, Vol. 9, No. 6, 2019, article no. 357, pp. 16–17).
It was especially interesting that the reactions seemed to correlate with the dull frosty white surface areas along the central structure lines on the sawn-faces when observed under the microscope at higher magnifications (indicated by black arrows in figure 4, A-3 and B-3). This observation was consistent with previous studies on freshwater cultured pearls (Spring 2019 Lab Notes, pp. 94–96).
Raman analysis of these areas showed they consisted of two CaCO3 polymorphs: aragonite and vaterite. Aragonite features were visible at 701, 704, and 1085 cm–1, while vaterite features were noted at 740, 750, 1075, and 1090 cm–1. Aragonite and vaterite in cultured FW pearls have previously been recorded (U. Wehrmeister et al., “Vaterite in freshwater cultured pearls from China and Japan,” Journal of Gemmology, Vol. 30, No. 7/8, 2007, pp. 399–412; A.L. Soldati et al., “Structural characterization and chemical composition of aragonite and vaterite in freshwater cultured pearls,” Mineralogical Magazine, Vol. 72, No. 2, 2008, pp. 579–592; H. Ma et al., “Vaterite or aragonite observed in the prismatic layer of freshwater-cultured pearls from South China,” Progress in Natural Science, Vol. 19, No. 7, 2009, pp. 817–820). Furthermore, calcite was also detected in pearl A, where peaks at 280, 714, and 1085 cm–1 were observed. The first two peaks were isolated and did not appear in association with any other peaks (groupings or doublets), proving they were another CaCO3 polymorph. Up until recently, calcite had not been reported in freshwater cultured pearls, though it was found in a small area of pearl A in this study and was also noted in a pearl discussed in a recent study (S. Eaton-Magaña et al., “Raman and photoluminescence mapping of gem materials,” Minerals, Vol. 11, No. 2, 2021, article no. 177, pp. 24–27).
Further advanced analysis of the cross sections’ trace element concentrations was conducted using laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS). The reddish orange fluorescent areas were shown to contain much higher Mg (1250 ppmw for pearl A and 2260 ppmw for pearl B) and lower Na (1210 ppmw for pearl A and 1310 ppmw for pearl B) concentrations than other areas of the samples not exhibiting the reaction (Mg lower than 77 ppmw and Na higher than 1930 ppmw). This result is consistent with those detailed in the literature (Soldati et al., 2008; Eaton-Magaña et al., 2021). The results seemed to correspond with the Raman data, and the different Mg and Na ratios helped to separate aragonite and vaterite, though it is difficult to differentiate calcite from either without performing Raman analysis.
Vaterite within the structure of NBC FW pearls is thought to be related to the biomineralization process that results following the tissue/mantle insertion (Wehrmeister et al., 2007), and its presence is a strong indicator of a cultured origin. These two unique pearls are a part of 28 Biwa pearl samples collected over various time frames. Since they are worthy of being singled out for discussion, this brief report is a precursor to a more detailed review of both pearls and the other Biwa pearl samples referenced.



