Natural Freshwater Pearls from Europe: Russia, Scotland, and Germany
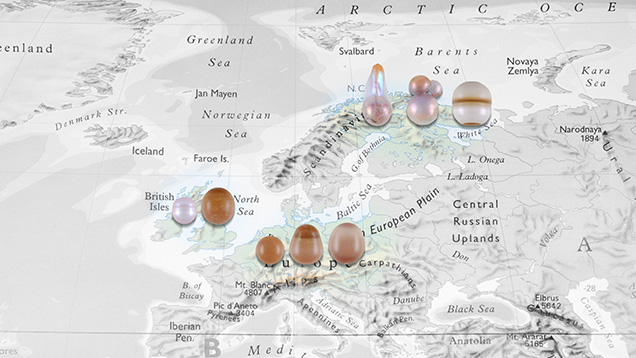
The Carlsbad laboratory received eight European freshwater (FW) pearls from coauthor ES for gemological and chemical characterization (figure 1). Although the exact locations and times of discovery were not recorded, all the samples were obtained directly by ES from reputable sources in each country. The first three samples are of Russian origin and were obtained by Russian fisheries biologist Valery Ziuganov during his studies of the Varzuga River, Kola Peninsula, in the 1990s (E. Strack, “European freshwater pearls: Part 1–Russia,” Journal of Gemmology, Vol. 34, No. 7, 2015, pp. 580–593; E. Strack, “Freshwater pearls from Russia,” Margaritologia newsletter, No. 11, 2018). Two samples from Scottish rivers were provided in 2004 by Cairncross Jewellers, the only company allowed to buy pearls from the fishers in Perth, Scotland. The last three are from Lüneburg Heath in northern Germany and were supplied around 1997 by a local family that had collected and fished for pearls over the decades. European FW natural pearls have reportedly been produced for centuries from mussels belonging to the Margaritifera species, which has been referred to as the “freshwater pearl mussel” (E. Strack, Pearls, Rühle-Diebener-Verlag, Stuttgart, Germany, 2006). Today, most natural FW pearls produced in North America and cultured FW pearls from Asia form in various mussel species within the Unionidae family. Both families are member of the Unionoidea superfamily.
The samples exhibited two different surface structures: nacreous and non-nacreous. Upon examination with a loupe and microscope, samples 1–4 (see table 1) exhibited nacreous surfaces, with fine overlapping platelets visible under high magnification. Surface wrinkling was also seen in single areas on samples 1 and 3, along with fine surface fractures; the wrinkled feature in each was previously noted as a characteristic of a FW environment (Strack, 2015, 2018). The nacreous samples ranged from 0.70 to 2.68 ct and measured from 4.77 × 4.33 mm to 7.08 × 6.72 mm. According to GIA classification procedures, their shapes were semi-baroque, baroque, and circled oval, while their bodycolors varied from light pinkish brown to light purplish pink and very light pinkish brown. Sample 3 showed a dull surface lacking luster and overtone, while the other three displayed orient and pink overtones.
Conversely, samples 5–8 exhibited a non-nacreous surface appearance and lacked overlapping platelets characteristic of most FW pearls. They ranged from 0.94 to 2.11 ct and measured from 5.12 mm to 7.32 × 6.98 mm. The shapes were uniformly round, oval, and drop, and all the samples were brown-yellow. At higher magnification, using strong illumination such as fiber-optic lighting, all the pearls displayed mosaic or cellular surface patterns of differing sizes and shapes. The small cells, which looked like pinpoint features in lower lighting conditions, resulted in a dimpled surface texture (figure 2). A cellular pattern was also observed in a small area on the base of sample 1. The non-nacreous appearance and cellular surface structures resembled those commonly observed on pen pearls from the Pinnidae family (N. Sturman et al., “Observations on pearls reportedly from the Pinnidae family (pen pearls),” Fall 2014 G&G, pp. 202–215), as well as some non-nacreous non-bead cultured pearls from the Pinctada maxima mollusk (A. Manustrong et al., “Known non-nacreous non-bead cultured pearls and similar unknown pearls of likely cultured origin from Pinctada maxima,” GIA Research News, 2019, https://www.gia.edu/gia-news-research/known-non-nacreous-non-bead-cultured-pearls). Non-nacreous natural pearls from Pteria species mollusks have also reportedly displayed hexagonal-like cellular patterns (S. Karampelas and H. Abdulla, “Black non-nacreous natural pearls from Pteria sp.,” Journal of Gemmology, Vol. 35, No. 7, 2017, pp. 590–592).
Under long-wave ultraviolet radiation (365 nm), the nacreous samples exhibited weak bluish yellow fluorescence over most of the surface, while a yellow reaction was observed on the limited yellowish brown areas. The non-nacreous samples exhibited uniform weak to moderate chalky yellow fluorescence similar to that reported for pen pearls (Sturman et al., 2014), but different from the strong orangy red fluorescence observed in non-nacreous Pteria pearls (S. Karampelas and H. Abdulla, 2017).
A Raman spectrometer was used to examine the surface composition of all the pearls, initially using a 514 nm argon-ion laser. Owing to the high background fluorescence observed in most of the samples, an 830 nm laser provided better peak resolution. Clear aragonite peaks at 702, 705, and 1085 cm–1 were recorded in all the samples (figure 3), and the results allowed the four non-nacreous pearls to be separated from pen pearls, which are normally composed of calcite (Sturman et al., 2014). Raman spectra collected using the 514 nm laser revealed weak polyene peaks at approximately 1125 cm–1 and 1512 cm–1 in samples 1, 3, and 4, indicating their colors were natural. Polyene peaks were not observed in samples 2 and 5–8, possibly due to the samples’ high fluorescence. The 830 nm laser is not helpful in resolving polyenic peaks. Surface observations did not reveal color modification in any of the samples.
UV-Vis reflectance spectra were collected from the smoothest and most homogeneously colored area of each sample in the 250–800 nm range. An absorption feature at about 280 nm that is usually observed in nacreous pearls was clearly seen in samples 1–4 but absent in samples 5–8. Samples 1, 2, and 4 showed reflection minima in the blue to yellow range, which corresponds with their predominantly pink hue. These results correlate with those in a previous study on naturally pink FW pearls of natural origin from the Mississippi River System in the United States (Summer 2019 GNI, pp. 282–285) and with reports on natural-color FW cultured pearls from Hyriopsis species mollusks (S. Karampelas et al., “Role of polyenes in the coloration of cultured freshwater pearls,” European Journal of Mineralogy, Vol. 21, No. 1, 2009, pp. 85–97; A. Abduriyim, “Cultured pearls from Lake Kasumigaura: Production and gemological characteristics,” Summer 2018 G&G, pp. 166–183). Owing to the very light color of sample 3, the spectrum obtained was nearly flat, as would be expected for white pearls, and hence no features of any significance were observed. The spectra obtained from samples 5–8 showed a lower reflectance than the four previously reported samples due to their more saturated colors. All the spectra showed similar patterns, with an incline from 400 to 750 nm in the visible region, comparable to the reported spectra of dark-colored pen pearls (Sturman et al., 2014) and non-nacreous non-bead cultured pearls from Pinctada maxima (Manustrong et al., 2019).
Table 1 shows the internal structures of the eight samples using real-time microradiography (RTX) and X-ray computed microtomography (µ-CT). Sample 1 and all the non-nacreous samples showed clear organic-rich concentric ring structures with faint radial features radiating outward from the center across the concentric rings. The radial structures are associated with cellular surface patterns. All three sections of sample 2 showed weak growth arcs corresponding to their shapes. Sample 3 exhibited a wavy concentric growth pattern together with a dark organic-rich patch, while µ-CT analysis revealed a small dark core in the structure. Sample 4 displayed the least amount of visible structure—only a few very faint growth arcs, even when examined using µ-CT. Cracks of varying lengths and degrees of visibility were present in the samples. The structures observed confirmed their stated natural origin. However, a smaller nucleus with a lighter gray core next to the main nucleus in sample 7 did raise some concerns. If the pearl was submitted to a gemological laboratory without any known provenance, the smaller structure could be mistaken for the lighter gray carbonate “seed” features sometimes found in saltwater non-bead cultured pearls (M.S. Krzemnicki et. al., “Tokki pearls: Additional cultured pearls formed during pearl cultivation: External and internal structures,” 32nd International Gemmological Conference, 2011, https://www.ssef.ch/wp-content/uploads/2018/01/SSEF_Tokki_pearls.pdf). However, the authors are not aware that this type of feature has ever been reported in non-bead cultured pearls from a FW environment.
Chemical compositions were initially analyzed by energy-dispersive X-ray fluorescence (EDXRF) spectrometry, and some of the samples were tested twice in different positions. Most samples contained relatively low manganese (Mn) levels (0 to 240 ppm) compared to the majority of natural and cultured FW pearls in GIA’s database. The usual Mn range observed is between 150 and 2000 ppm. Samples 1, 3, and 6–8 showed Mn concentrations below 50 ppm. It was interesting to note that in sample 5, Mn was absent at one end but present (170 ppm) at the opposite end. Additionally, the samples with low Mn levels (<50 ppm) did not show visible reaction under X-ray excitation (H. Hänni et al., “X-ray luminescence, a valuable test in pearl identification,” Journal of Gemmology, Vol. 29, No. 5/6, 2005, pp. 325–329), as would be expected for such low traces of the element. Strontium (Sr) contents were relatively low (<1000 ppm) in all the samples, which is characteristic of FW origins. However, the very low concentration of Mn and lack of X-ray fluorescence reactions could create some doubts and lead to some misidentifying them as saltwater (SW) pearls.
Detailed trace element concentrations were analyzed using laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) with the same parameters used in previous FW pearl studies (A. Homkrajae et al., “Provenance discrimination of freshwater pearls by LA-ICP-MS and linear discriminant analysis (LDA),” Spring 2019 G&G, pp. 47–60; Summer 2019 GNI, pp. 282–285). At least three ablation spots were tested on each sample, and the results for the 22 elements selected are shown in table 2. The five elements (sodium, magnesium, Mn, Sr, and barium) found to be useful discriminators in the differentiation of FW from SW pearls and in classifying FW pearls from different sources are bolded in the table. Mn and Sr contents obtained were consistent with the EDXRF results.

All the samples contained low Mn levels (<500 ppm), separating them from the majority of FW pearls previously studied (figure 4; see Homkrajae et al., 2019; Summer 2019 GNI, pp. 282–285). The ternary diagram of relative percentages between Ba, Mg, and Mn that was used to verify the growth environment conditions of the questionable FW pearls in previous studies (Homkrajae et al., 2019; Summer 2019 GNI, pp. 282–285) was used once again to confirm the formation environment of the six samples containing low levels of Mn (below 100 ppm). All six samples plotted alongside those of the FW pearls previously studied, confirming their FW origin. These results also help to distinguish non-nacreous samples from saltwater non-nacreous non-bead cultured pearls produced by Pinctada maxima (Manustrong et al., 2019). The values of these five discriminant elements, as well as those for lead (Pb), correspond with the chemical results reported on natural FW pearls produced by Margaritifera mollusks found in the Spey River, Scotland (S. Karampelas et al., “Chemical characteristics of freshwater and saltwater natural and cultured pearls from different bivalves,” Minerals, Vol. 9, No. 6, 2019, p. 357). The Sr-Ba concentration plot is also shown in figure 5. This result supports the observation made by Karampelas et al. (2019). Furthermore, the pearls from each European locality could be separated from each other in this plot, though it should be noted that this was based on a very limited number of known samples.
For centuries, the freshwater mussel species Margaritifera was abundant in the rivers and streams of Europe, and the pearls produced were part of European culture and history. The American native mussel species followed a similar path. However, environmental changes, and the impact of pollution from industry and agriculture have damaged ecosystems and directly affected the mussels’ habitat and lifespan (Strack, 2006). Freshwater pearl fishing in Europe is currently prohibited or under regulation due to the decline of mussel populations. Studying these rare European FW natural pearls provided valuable data that has enlarged GIA’s identification database, offering a useful reference for the gemological community.



