Detection of Color Treatment and Optical Brightening in Chinese Freshwater “Edison” Pearls

ABSTRACT
Color origin is an important part of pearl identification for gemological laboratories. Freshwater cultured pearls can exhibit a wide range of colors, which can be either naturally formed or artificially modified by post-harvest treatments. The most common color treatments are dyeing and irradiation. In this project, a group of 23 freshwater “Edison” pearls (a trade name referring to near-round to round freshwater bead cultured pearls invented and produced by Zhejiang Grace Pearl Jewellery Co Ltd in China), reportedly with a mix of natural and treated colors, were analyzed using various gemological and advanced instrumental techniques in order to distinguish their color origin. A number of techniques, including microscopic observation, long-wave UV fluorescence observation, long-wave and short-wave fluorescence spectroscopy, Raman spectroscopy, and trace element analyses, were used in this study. Of the 23 freshwater samples, 19 have been confirmed as bead cultured pearls and 4 have been identified as non-bead cultured pearls. In addition, seven samples have been identified as color-treated, while five of the remaining 16 samples were also identified as being optically brightened (a process commonly used on freshwater cultured pearls to improve appearance factors such as luster). Our results proved that color treatment in these freshwater pearls could be confidently identified using this combination of techniques, as could optical brightening processes that are routinely applied to untreated, naturally colored freshwater cultured pearls post-harvest.
Introduction
Currently, China is the world’s largest producer of cultured pearls, and the majority are cultured in freshwater environments such as rivers, lakes, and ponds (Cartier and Ali, 2013; Zhu et al., 2019). Over the last several decades, advances in freshwater culturing techniques have also significantly improved the quality of Chinese freshwater cultured pearls on the market, such as Ming pearls and “Edison” pearls, to the point where these round bead cultured products can rival saltwater cultured pearls such as akoya, South Sea, and Tahitian pearls (Scarratt et al., 2000; Akamatsu et al., 2001; Fiske and Shepherd, 2007; Hänni, 2011; Karampelas, 2012; Zhou and Zhou, 2015; Otter et al., 2017; Li et al., 2018; Bui et al., 2019). The term “Edison pearl” specifically refers to the bead cultured pearls produced by Grace Pearls of China, while “Ming pearl” is a more generic term that describes high-quality bead cultured freshwater pearls produced by other producers. Recently, stricter enforcement of the Chinese government’s environmental policies has impacted the output of freshwater cultured pearls, as many farms were closed down due to pollution problems (Zhou and Lu, 2018). Increasing demand for high-quality freshwater cultured pearls in China and around the world will likely shift the production toward larger, rounder, and more attractive colored pearls in the future.
Detection of color treatment is an important part of pearl identification in gemological laboratories, both for saltwater and freshwater pearls (Elen, 2001; Wang et al., 2006; Karampelas et al., 2007, 2011; Zhou et al., 2012, 2016; Tsai and Zhou, 2020). Freshwater cultured pearls, in particular, can exhibit a wide variety of coloration, including white, cream, yellow, orange, brown, pink, and purple. In addition to various artificial color modification treatments, freshwater cultured pearls are also routinely processed after harvest by bleaching and optical whitening and brightening using optical brightening agents (OBA) (Zhou et al., 2020). These two processes have very different mechanisms: Bleaching removes, or reduces the concentrations of, the color-causing pigmentations within pearls, while optical brighteners add an artificial optical effect to pearls, making them look less yellow by increasing the overall amount of blue light emitted due to these chemicals’ blue fluorescence properties. It is important to be able to differentiate naturally colored pearls from their treated counterparts from a gemological laboratory point of view, which is the underlying reason for this study.
The global pandemic that started at the beginning of 2020 caused little disruption to the production of freshwater cultured pearls in China, as the harvest usually takes place at the end of the year and the Chinese government’s fast control of the virus minimized the impact. However, the export of freshwater cultured pearls has been significantly affected, and dealers are trying to boost domestic sales by emphasizing a strong online presence (Zhou and Chen, 2020). The parent company of Grace Pearl, Zhejiang Dongfang Shenzhou Pearl Group, continues to develop new freshwater pearl products such as the “China Red” (orange and red variety of Edison pearls) and “mini-Akoya” (small white-colored bead cultured pearls), which have gained popularity in the market (Wong, 2020). The capability to accurately detect color treatment can further increase consumer confidence when purchasing these high-quality freshwater pearl products, such as the loose pearls and necklace shown in figure 1.
MATERIALS AND METHODS
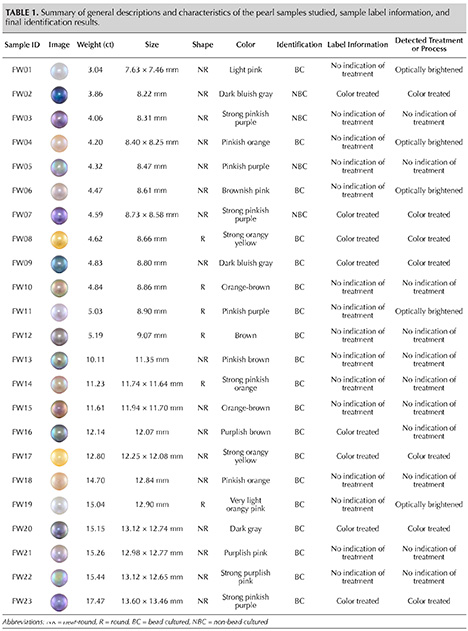
The present study focuses on the identification of treated freshwater cultured pearls from a group of 23 variously colored samples (figure 2). These pearls were supplied by Zhejiang Grace Pearl Jewellery Co Ltd in China, the original producer of so-called “Edison” pearls. The 23 undrilled samples range in size from 7.63 × 7.46 mm to 13.60 × 13.46 mm. These were reportedly grown in the cultured pearl farms owned by the company. Such pearls are usually bead nucleated using Hyriopsis cumingii or hybrids of the Chinese Hyriopsis cumingii and the Japanese Hyriopsis schlegelii mussels. Some of these samples were further treated post-harvest by the producer to enhance their appearance attributes such as bodycolor, overtone, or luster. These samples contained initial labels stating “treated” and “natural,” however, no information of optical brightening was mentioned. We regarded these pearls as “unknown” samples and performed the same testing procedures regardless of the labels.

All samples were examined using various standard gemological techniques, including visual observation under a gemological microscope, microscopic imaging using a Nikon SMZ 1500 stereomicroscope, observation of the fluorescence reactions under a conventional 5-watt long-wave UV (LWUV) (365 nm) lamp, and real-time microradiography (RTX) analysis using a Pacific X-ray Imaging (PXI) GenX-90P system with a 4-micron microfocus, 90 kV voltage, and 0.18 mA current X-ray source, fitted with a PerkinElmer 4”/2” dual-view flat panel detector.
A number of advanced instrumental techniques were also applied in this study. These include Raman spectroscopy using a Renishaw inVia Reflex micro-Raman spectrometer system with a 50× magnification Leica objective lens and a 514 nm argon-ion laser (scanning range from 100 to 1600 cm–1), ultraviolet (UV) fluorescence spectroscopy under both long-wave UV excitation with a 385 nm LED light source (M385L2, Thorlabs) and an Avantes AvaSpec-Mini spectrometer (scanning range from 400 to 950 nm), and short-wave UV excitation with a 275 nm LED light source (M275L4, Thorlabs) and an Avantes AvaSpec-Mini spectrometer (scanning range from 300 to 1100 nm). Trace element analyses were conducted using a Thermo Scientific ARL Quant’x energy-dispersive X-ray fluorescence (EDXRF) spectrometer (calibrated with MACS-1 and MACS-3 carbonate standards) and a Thermo Fisher Scientific iCAP Qc inductively coupled plasma–mass spectrometry, coupled with an Elemental Scientific NWR213 laser ablation system (LA-ICP-MS) (three spots on each sample’s surface, 55 μm spot size). More details on these gemological and instrumental methods for the application of pearl identification can be found in previously published papers (Zhou et al., 2017, 2019; Nilpetploy et al., 2018; Homkrajae et al., 2019; Sturman et al., 2019; Tsai and Zhou, 2020).
RESULTS
Gemological Observations. All 23 samples were either round or near-round, ranging from 7.63 × 7.46 mm to 13.60 × 13.46 mm and from 3.04 ct to 17.47 ct. Their bodycolors exhibited various hues such as pink, gray, purple, orange, yellow, or brown in different degrees of tones and saturations. Standard real-time microradiography analysis revealed that the majority contained a drilled bead nucleus, as expected for Edison pearls. However, four of the 23 samples showed irregular linear internal growth features found in many freshwater non-bead cultured pearls (Scarratt et al., 2000; Krzemnicki et al., 2010), although their external appearances were indistinguishable from the rest of the samples in this group. The four non-bead cultured pearls were FW02, FW03, FW05, and FW07, which were all relatively small (8–9 mm). Technically these pearls were not considered Edison pearls, and they were probably formed by accident when the bead nuclei were rejected by the mussels during growth.

Under a conventional gemological microscope, all of the pearls appeared to have even color distribution. Minor color concentration spots were found on the surface of five samples (FW02, FW07, FW08, FW17, and FW23), yet they were rather sporadic and difficult to see because they were small and faint. Nevertheless, FW08 and FW17 appeared to be color treated based on their bodycolors, possessing “golden” colors that looked very unnatural to the unaided eye and under the microscope. In the authors’ experience, such color appearances are almost nonexistent in freshwater cultured pearls. Furthermore, while freshwater pearls may exhibit varying degrees of fluorescence under LWUV excitation based on their bodycolor intensities (usually weaker fluorescence with darker or more saturated colors), the strong, spotty, and more bluish fluorescence found in five of the samples (FW01, FW04, FW06, FW11, and FW19) suggested that they had been optically brightened. The remaining pearls were either inert under LWUV excitation or exhibited an even yellowish or whitish reaction (figure 3). In order to confidently identify color treatments and optical brightening processes, additional advanced testing techniques were employed.

Advanced Testing Results. Raman spectroscopy has long been one of the most important spectroscopic techniques used in identifying natural pigments in some mollusk shells and pearls, in addition to confirming their major mineral constituents (mainly aragonite but also rarely calcite and vaterite) (Karampelas et al., 2007; Soldati et al., 2008a,b; Karampelas et al., 2009; Bersani and Lottici, 2010; Wehrmeister et al., 2011; Karampelas et al., 2019a,b). It is particularly useful in detecting natural polyenic molecules in freshwater pearls, thus enabling the separation of naturally colored and treated-color pearls. Raman spectroscopic analysis with a 514 nm argon-ion laser revealed aragonite peaks at 703/705 cm–1 (doublet) and a main peak at 1086 cm–1 due to the ν4 in‐plane bending mode and the ν1 symmetric mode, respectively, for all samples. However, six of the 23 pearls (FW07, FW08, FW09, FW17, FW20, and FW23) showed significantly reduced aragonite peaks and higher fluorescence background. Besides aragonite Raman peaks, strong polyene peaks in the approximate ranges of 1125–1134 and 1508–1527 cm–1 due to ν2 vibration from stretching of C–C single bonds and ν1 vibration from stretching of C=C double bonds were observed for 14 of the 23 samples in this study (figure 4). In addition, minor bands at around 1017 and 1298 cm–1 that are also associated with polyene pigments were found on these pearls. Upon closer examination, the main polyenic peaks can be divided into two groups with subtle Raman shift differences: one group exhibiting peaks between 1125–1128 cm–1 and 1508–1511 cm–1 (FW03, FW05, FW10, FW11, FW12, FW13, FW15, FW16, and FW22), while the other group exhibits peaks between 1133–1134 cm–1 and 1526–1527 cm–1 (FW04, FW06, FW14, FW18, and FW21). The rest of the pearls showed high background fluorescence or lacked any pigment peaks (figure 5), indicating their artificial color origin, except FW01 and FW19, which had very light bodycolors to start with. Based on Raman results, seven colored pearls in this group have been identified as treated (FW02, FW07, FW08, FW09, FW17, FW20, and FW23). This also matched with the microscopic observations discussed in the previous section, where the five pearls showing minor surface color concentrations were all confirmed by Raman spectroscopy as color treated.

While Raman spectroscopy was effective in detecting the organic pigments in naturally colored freshwater pearls, further trace element analyses using EDXRF and LA-ICP-MS techniques provided additional information on the nature of the dye materials as they were particularly useful in detecting the artificial inorganic pigments. Five of the seven treated pearls (FW02, FW07, FW09, FW20, and FW23) were found to contain an abnormally high amount of silver (Ag) on their surfaces with both analytical techniques, indicating the dark colors were modified using silver nitrate. The concentrations of Ag found on the surfaces of these pearls ranged from 306 to 2850 ppm, while no detectable amount of Ag was found on the rest of the samples. The other two treated-color “golden” pearls (FW08 and FW17) may have been modified by organic dyes, as neither EDXRF nor LA-ICP-MS detected any anomalies in their trace element concentrations. However, certain elements such as F, Cl, P, S, N, and C cannot be properly measured by LA-ICP-MS due to their high first ionization energy and interferences. The 16 untreated pearls were also tested with EDXRF and LA-ICP-MS; none of them contained Ag or any other unusual trace element concentrations.


More recently, short-wave UV fluorescence spectroscopy has been applied to pearls in order to check the fluorescence from their organic content. The amino acid tryptophan is a major UV fluorophore found in nacre. It has an absorption band at around 280 nm and can subsequently fluoresce at around 320–360 nm, a key indicator of whether the nacre has been treated or processed, as many of the treatments and processes can reduce this fluorescent feature (Lakowicz, 2006; Hiramatsu and Nagai, 2010; Tsai and Zhou, 2020, 2021). It was found that treated or heavily processed pearls would exhibit reduced nacre fluorescence intensity in this region. A quick check on all of the samples agreed with the Raman results, where all of the seven color-treated pearls emitted very low fluorescence (<25 counts per millisecond under the current instrument setup) compared to the 14 naturally colored pearls (>70 counts per millisecond) (figure 6). The two light-colored pearls (FW01 and FW19) also showed lower nacre fluorescence and an additional strong peak around 420–430 nm due to an optical brightening process (Zhou et al., 2020). However, SWUV fluorescence spectroscopy is not an ideal method for detecting optical brightening, as these chemicals typically produce their maximum fluorescence signal under LWUV excitation. Figure 7 shows the pearls’ fluorescence under long-wave UV excitation; five pearls exhibited more prominent fluorescence near 420–430 nm, suggesting that they were processed by optical brighteners. These are the two light-colored pearls (FW01 and FW19), as well as the three natural-color pearls (FW04, FW06, and FW11). The result is also consistent with visual observation under LWUV excitation, as all five pearls displayed strong bluish fluorescence that was readily visible (figure 2). A summary of all the samples, how they were labeled by the vendor, and their final identification results is shown in table 1.
DISCUSSION
Freshwater Edison pearls have emerged as a popular type of cultured pearl in the market during the past decade. Their near-round shape and variety of attractive non-neutral fancy bodycolors represent a significant improvement in Chinese freshwater cultured pearl quality. Many of these Edison pearls exhibit intense bodycolors in the three main hue categories: purple, pink, and orange. These pearl colors are affected predominantly by the donor oyster’s nacre color, which has been known to be genetically determined and inherited (McGinty et al., 2010; Karampelas and Lombard, 2013; Ky et al., 2014; Wang et al., 2020). For freshwater pearls, it is also known that polyenic pigments (such as unmethylated and partially methylated polyenes) containing various chain lengths of alternating double and single carbon-carbon bonds are responsible for color appearance (Soldati et al., 2008a; Karampelas et al., 2007, 2009, 2019a). In addition to the effect of genetic inheritance, it is also possible that various carotenoid pigments similar to the natural pigments found in freshwater pearls are used as food additives during the culturing process to enhance the pearls’ colors, a common practice that can be found in other aquatic industries (Liao and Miao, 2013; Pereira da Costa and Miranda-Filho, 2020). These dietary supplements will be absorbed and metabolized by mussels growing in the culturing farms, which in turn can be transformed into their own natural pigments. However, this is not considered a form of treatment, and whether the samples obtained for this study underwent this practice is not known to the authors.
Our study suggested that both color treatment and optical brightening have been applied to fancy-color freshwater Edison pearls, and their identification can be achieved by a combination of conventional gemological methods (microscopic observation and long-wave fluorescence observation) and advanced instrumental techniques (Raman spectroscopy, trace element analyses, and long-wave and short-wave UV fluorescence spectroscopy). It is worth noting that conventional UV-Vis reflectance spectroscopy is a very effective method to identify color treatment in golden or dark-colored saltwater pearls (Elen, 2001; Wang et al., 2006; Karampelas et al., 2011; Zhou et al., 2012, 2016). However, the identification of color treatment in freshwater pearls using UV-Vis reflectance spectroscopy is more challenging and less effective in our experience with these samples (results not shown). Freshwater pearls can exhibit many different colors with various tones and saturations, without easily identifiable characteristic absorption peaks such as the 700 nm absorption band found in Tahitian pearls. Among all the techniques, Raman spectroscopy can provide positive identification of natural color origin by the detection of polyenic pigments found on the surface of the pearls. The differences in polyene-related peak positions at 1125–1134 and 1508–1527 cm–1 between two groups of samples (purplish and brownish hues vs. orangy and pinkish hues) are most likely due to the different carbon-carbon single- and double-bond chain lengths, with longer chain polyenes producing lower-frequency Raman bands (Hedegaard et al., 2006). On the other hand, trace element analyses such as EDXRF and ICP-MS can effectively identify certain color-treated pearls, especially those treated with silver nitrate. Subsequent short-wave UV fluorescence spectroscopy can further separate natural and treated pearls based on their nacre fluorescence under UV excitation, confirming the results obtained by other techniques. One sample in our study (FW16) was initially labeled as treated when it was received by us. However, both gemological and advanced testing results confirmed that this pearl’s color was of natural origin, and there were no indications of optical brightening. While we cannot rule out any other undetectable processes that could be applied to pearls, we believe this is most likely a result of sample mislabeling or misplacement from the source.
White pearls and those with very light hues, especially akoya and freshwater cultured pearls, are routinely processed or treated by various methods after harvest. Bleaching is the most prevalent and is often used in addition to maeshori treatment, an umbrella term for various types of luster enhancement (Otter et al., 2017). Optical brightening agents are also used sometimes in conjunction with bleaching/maeshori to further enhance the appearance of the pearls. This additional process can be detected using long-wave fluorescence spectroscopy, as the brightening agents will fluoresce at a specific wavelength (around 420–430 nm) under long-wave excitation (Zhou et al., 2020). While some aspects regarding the impact of optical brightening on fancy-color freshwater pearls remain inconclusive, this study revealed that some naturally colored freshwater pearls have also been subjected to this process. Although their color is not affected by the optical brightening, their luster could benefit from this process, which can increase the pearls’ reflection in the visible spectrum. Finally, we did not observe any optical brightening process applied to color-treated pearls in this group of samples, based on both visual observation of their fluorescence under LWUV and spectroscopic results.
CONCLUSIONS
This study proves that GIA’s current testing protocols are effective in detecting color treatments in freshwater cultured pearls. The color origin of fancy-color Edison pearls can be confidently determined with a combination of conventional gemological tests and advanced analytical methods. Raman spectroscopy, trace element analyses, and short-wave UV fluorescence spectroscopy all proved useful in separating natural and treated colors. In addition, some naturally colored Edison pearls in the trade have been subjected to an optical brightening process. This treatment can also be identified using long-wave UV fluorescence observation and spectroscopic methods, through the detection of characteristic fluorescence peaks at around 420–430 nm caused by the brightening agents. While these samples only represented a small pool of this unique type of cultured pearl product, the treatment and optical brightening features are quite characteristic and could be applied to other types of freshwater cultured pearls in the market such as Ming pearls and traditional non-bead cultured pearls.
.jpg)


