Hydrogen and Oxygen Stable Isotope Ratios of Dolomite-Related Nephrite: Relevance for Its Geographic Origin and Geological Significance
ABSTRACT
Hydrogen and oxygen stable isotope ratios of dolomite-related nephrites around the world were studied using data from the literature (n = 120). These isotope ratios are highly effective for discriminating dolomite-related nephrites from the four most important origins worldwide. Nephrite from Vitim in Russia has the lowest isotope ratio values reflected in δ2H and δ18O values, followed by Chuncheon in South Korea and then Xinjiang Uyghur Autonomous Region in China. Nephrite from Sanchakou in the Qinghai Province of China has the highest values. Other occurrences are characterized by high δ18O values similar to or higher than those of samples from Sanchakou. The differences are derived mainly from the ore-forming fluids. Vitim and Chuncheon isotope ratio values were mainly affected by meteoric water (rainwater, lake water, seawater, river water, glacial water, and shallow groundwater). Xinjiang nephrite-forming fluids were mixtures of magmatic hydrothermal fluids (able to be modified by metamorphism) and meteoric water. The hydrothermal fluids forming the Qinghai, Luodian, Dahua, and Xiuyan nephrites underwent some metamorphic alteration or regional metamorphism.
INTRODUCTION
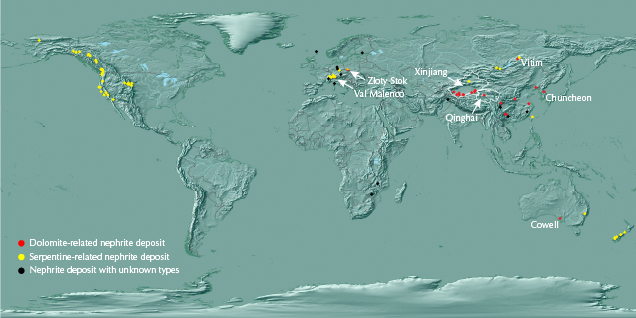
Nephrite is a near-monomineralic rock composed of tremolite-actinolite, Ca2(Mg,Fe)5Si8O22(OH)2. It occurs worldwide (figure 1) and is classified as dolomite-related or serpentine-related according to the different parent rocks and ore-hosting rocks, and both types form by metasomatism (Yui et al., 1988; Tang et al., 1994; Yang and Abduriyim, 1994; Harlow and Sorensen, 2005; Burtseva et al., 2015). The large and well-known dolomite-related nephrite deposits are distributed in the Xinjiang Uyghur Autonomous Region (hereafter abbreviated as Xinjiang) of China, Qinghai Province of China, Siberia in Russia, and Chuncheon in South Korea (figure 1). Data from smaller-scale deposits such as Val Malenco in Italy and Złoty Stok in Poland are also used in this study (figure 1). The rest of the data were collected from nephrites produced at multiple small-scale sources in China: Xiuyan, Tanghe, Dahua, and Luodian (figure 2).

With nephrite jade, a premium is placed on geographic origin since the gem’s cultural significance differs by location. It is possible to have an opinion on the origin of a small amount of nephrite by simple visual examination, since some varieties with unique gemological characteristics such as color, luster, and transparency have mainly occurred in specific deposits. In Xinjiang, for example, high-quality white primary nephrite occurs in Qiemo County.1 There it is commonly associated with brown nephrite (figure 3A, rough with white core and very thick brown rind). The brown is a color seldom found in nephrite from other deposits in Xinjiang. The highest-quality white primary nephrite (figure 3B, white plate) mostly comes from the Hetian region and Qiemo County. Placer nephrite (figures 3C and 3D, pendants with figures carved out of brownish red skin) occurs in the Yulongkashi River and Kalakashi River basins. A considerable quantity of primary nephrite from Ruoqiang County features a yellow color component (figure 3E, greenish yellow fish) that is absent from other samples. Black nephrite (figure 3F, bangle bracelet) colored by graphite, on the other hand, mainly occurs in the Hetian region and has not been found in Qiemo County or Ruoqiang County. However, the origin determination of a tremendous amount of dolomite-related nephrite cannot be solved by this simple observation. Previous researchers used trace elements combined with appearance to identify geographic origin and obtained some informative results (Zhong et al., 2013; Luo et al., 2015). Unfortunately, rigorous and scientific determination of geographic origin is still not available.
1This paper uses Chinese pinyin to express all Chinese location names involved, and the corresponding commonly used English names are listed in table 1.

Hydrogen and oxygen isotope ratio values (see box A), which might vary for the same gemstone from different regions due to diverse ore-forming environments and models, can be used for geographic origin determination (Giuliani et al., 1998, 2000, 2005, 2007). A mass spectrometer is needed to determine the isotope ratio values (see box B). The spot produced by secondary ion mass spectrometry (SIMS), laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS), and laser ablation inductively coupled plasma time-of-flight mass spectrometry (LA-ICP-TOF-MS) for stable isotope analysis of gemstones can be restricted to craters of 10–100 m in diameter and a few angstroms to microns deep (Giuliani et al., 2000, 2005; Abduriyim and Kitawaki, 2006; Wang et al., 2016, 2018). The craters produced are very small, to the point of not being noticeable without magnification. This permits the method to be applied to gemstones and historical antiques (Giuliani et al., 2000, 2005).
|
Box A: Introduction to Oxygen and Hydrogen Stable Isotope Ratios |
|
Atoms with an equivalent atomic number (i.e., atoms of the same element) can differ from one another in their number of neutrons. For example, 18O has 8 protons and 10 neutrons, and 16O has 8 protons and 8 neutrons; 2H, also known as deuterium (D), has 1 proton and 1 neutron, while 1H has 1 proton and no neutrons. Such atoms with the same number of protons but different numbers of neutrons are defined as isotopes. The mass difference inherent from divergent neutrons causes isotopic fractionation, which occurs as the isotopes of an element are distributed between two substances or phases in differing ratios in a given system. This process can be affected by temperature, equilibrium or kinetic processes, and other physiochemical processes. The isotope fractionation will reach and maintain equilibrium unless conditions change. Therefore, isotope abundance can be used as a tracer to reveal certain geochemical processes in geological bodies. Isotope ratio, defined as the measured relative abundance of a heavy isotope to its lighter counterpart (e.g., 18O/16O and 2H/1H), is typically used rather than the isotope abundance itself. The isotopic fractionation factor (a) is introduced to represent the extent of fractionation of isotopes between two phases. It is defined as the ratio of isotope ratios in one phase to the other coexisting phase. For example, in a system consisting of phase A and phase B, the oxygen isotope fractionation factor can be defined as 
The isotopic fractionation factor is always a function of temperature, which can be obtained by theoretical calculation or experiment (Graham et al., 1984; Zheng, 1993, 1995). Both oxygen and hydrogen isotope ratios are also reported in so-called delta notation given in terms of per mil (‰). In other words, the delta value 
and 
in which 18O/16O and 2H/1H are the isotope ratios defined above. Values of delta > 0 indicate that relative to the standard samples, the tested sample has a higher heavy isotope abundance, and a negative delta value indicates a higher light isotope abundance. International general isotope standards are issued by the International Atomic Energy Agency (IAEA) and the U.S. National Institute of Standards and Technology (NIST). The delta values of hydrogen and oxygen isotopes are calculated using the value for Standard Mean Ocean Water (SMOW), which has 2H/1H of (155.76 ± 0.10) × 10–6, 18O/16O of (2005.20 ± 0.43) × 10–6, and 17O/16O of (373 ± 15) × 10–6. Other hydrogen isotope standard samples include SLAP, GISP, NBS-22, and NBS-30. |
Geographic origin discrimination of nephrite by isotopes is seldom reported, even though many hydrogen and oxygen isotope ratio studies on this material have been carried out (table 2). By summarizing and analyzing all available hydrogen and oxygen isotopic data of dolomite-related nephrites worldwide from published references, this study discusses the geographic origin discrimination based on the relationship between the characteristics of nephrite and its formation environment.

WHY DO ISOTOPE RATIOS MATTER TO GEMOLOGISTS?
The application of isotopes has gradually attracted the attention of gemologists (Wang et al., 2016). In addition to hydrogen and oxygen isotope ratios, which can help determine the geographical origins of corundum and emerald (Giuliani et al., 1998, 2000, 2005, 2007; Wang et al., 2019), carbon isotopes are considered useful in identifying natural and synthetic diamonds (Wang et al., 2014), and radioactive isotopes have also been used to determine the ages of gemstones (Link, 2015).
Traditional methods using the parameters of inclusions, optical characteristics, and trace elements are often not enough to solve the problems of geographic origin determination of nephrite. Isotopic analysis has provided geochemical and chronological information for all sorts of geological samples: Stable isotopes can be used to study gemstone origin (source materials, formation process, and geographical localities), whereas radioactive isotopes can be utilized to determine the formation ages. The stable isotope study of dolomite-related nephrite in our works, together with previous studies on corundum and emerald (Giuliani et al., 1998, 2000, 2005, 2007; Wang et al., 2019), show that the geographic origin characteristics of isotopes in gemstones can be explained from their formation environment and formation process. Thus, relative isotopic abundances are reliable parameters for determining geographic origin and offer a sound complement to traditional methods.
DATA AND CALCULATION
In all, 120 sets of hydrogen and oxygen isotope data (some lacking hydrogen data) for dolomite-related nephrites were collected from all known related published studies, from a variety of researchers (table 2 and figure 4), to illustrate geographic origin discrimination with stable isotopic ratios.

Hydrogen and oxygen isotope delta values of nephrite can be used to calculate the corresponding values of its formation fluids. Hydrogen isotope fractionation of tremolite relative to water is not affected by temperature in the approximate range of 350° to 650°C (Graham et al., 1984), and thus

while oxygen isotope fractionation (Zheng, 1993, 1995) can be expressed as

In both equations, αTr–H2O is the isotopic fractionation factor (see box A) between the nephrite and its formation fluid, and T is the absolute temperature (K) of the nephrite-forming system. The nephrite formation temperature is confined to approximately 223°–425°C, especially near 350°C (Tang et al., 1994; Yui and Kwon, 2002; Chen et al., 2014; Liu et al., 2016), by methods using the homogenization temperatures of tremolite fluid inclusions (Liu et al., 2011a; Chen et al., 2014), the combination of the pyrite decrepitation temperature and calcite homogenization temperature (Wang et al., 2007; Xu and Wang, 2016), the mineral assemblage (Yang, 2013), and isotopes (Yui et al., 1988). Thus, the value of 350°C was used to calculate the isotopes of fluids from which nephrite forms.
Both the isotope fractionation factor αTr–H2O and delta values (δ18O, δ2H) are defined after isotope ratios (18O/16O, 2H/1H) of nephrite and its formation fluids (see box A). Thus, the delta values of the nephrite-forming fluids (δ18OTr–H2O, δ2HTr–H2O; table 2) can be calculated from the delta values of corresponding nephrite, which is acquired by isotope determination (see box B).

GEOGRAPHIC ORIGIN CHARACTERISTICS
Vitim in Russia, Chuncheon in South Korea, and Xinjiang and Qinghai in China are the four most important dolomite-related nephrite source areas. The relative abundances of the hydrogen and oxygen isotopes of nephrites from these regions differ significantly (figure 4). In particular, oxygen isotope δ18O values (see figure 4 and table 2) range from −20.0‰ to −14.6‰, −9.9‰ to −8.2‰, 0.5‰ to 7.9‰, and 11.4‰ to 12.6‰, respectively, without any overlap. Cowell in Australia is considered another large dolomite-related nephrite deposit but is seldom studied. The only δ2H– δ18O data (see figure 4 and table 2) fall within the range of Xinjiang placer nephrite; nevertheless, the δ2H values are significantly higher than those of Xinjiang primary nephrite.
The nephrites from Xinjiang, distributed in a belt longer than 1300 km, show convergent hydrogen and oxygen isotopic characteristics. The isotope delta values of their primary dolomite-related nephrites are covered by placer ones (figure 4).
Samples from some relatively small deposits such as Xiuyan in Liaoning Province, Złoty Stok in Poland, and Dahua in Guangxi Province (figure 4) show slightly higher δ2H values than those of Qinghai nephrite and Xinjiang primary nephrite. Their ranges of δ18O values cover that of Sanchakou samples but do not overlap with Xinjiang primary nephrite. Fortunately, nephrites from these three regions typically have their own distinct appearances. Luodian nephrite from Guizhou has notably higher δ18O values than the others (no δ2H value data have been collected). In recent years, secondary nephrite has been found in the Tanghe River in Hebei Province. It is speculated to be a dolomite-related nephrite according to the regional geology, field observation, and petrographic analysis (Chen et al., 2014). Its hydrogen and oxygen isotope ratios are completely isolated from others in the plot of δ2H– δ18O (figure 4) by low δ2H and high δ18O values.
NEPHRITE-FORMING FLUIDS FROM MAGMATIC WATER AND METEORIC WATER
Fluids containing gases, liquids, and silicate compositions always occur as the most active parts of geological processes. They are composed mainly of H2O, CO2, NaCl, metal components, silicate compositions, and organic matter. The fluids that correspond to nephrite formation are hydrothermal fluids, which refer to gas-liquid two-phase systems having their own temperatures and pressures. Hydrothermal fluids are released from magma (magmatic fluids) or metamorphism (metamorphic fluids) due to changes in temperature and pressure. They also can be meteoric waters (including rainwater, lake water, seawater, river water, glacial water, and shallow groundwater) heated by geological processes.
The original characteristics of the hydrogen and oxygen isotopes of nephrite mainly result from the ore-forming fluids. The calculated δ2HTr–H2O and δ18OH2O values of hydrothermal fluids forming the Vitim and Chuncheon nephrites plot near the Craig line2 (figure 5), indicating that their predominant ore-forming fluids were meteoric waters in an environment with a high fluid/rock ratio (Yui and Kwon, 2002; Burtseva et al., 2015).
2The Craig line, also referred to as the meteoric water line, represents the relationship between δ2H and δ18O of meteoric water—i.e., δ2H = 8 δ18O+10 (Craig, 1961). The kaolinite line (Zheng and Chen, 2000) shown in figure 5 represents the relationship between δ2H and δ18O of kaolinite in weathering profile (i.e., δ2H = 7.5 δ18O–220). Most of the soil samples in nature fall on or near the kaolinite line.

For the Xinjiang nephrite, magmatic fluid, meteoric water, and metamorphic water are all possible candidates for the ore-forming fluids (figure 5), and a low fluid/rock ratio is indicated (Yui and Kwon, 2002; Liu et al., 2011a, 2011b, 2016). The δ18OH2O values of the nephrite-forming fluids for Alamasi nephrite, which occurs in granite-dolomite contact zones (Liu et al., 2010, 2011a), decrease in the contact zone in the order of granite → nephrite → wall rock. The δ18OH2O values of magmatic fluids, seldom influenced by crustal rocks during intrusion, should equal the high values of the Xinjiang nephrite-forming fluids (figure 5). The δ18Odol values of wall rock are far lower than those of common carbonates of sedimentary origin, at only 6.1‰ (Wan et al., 2002). Then, the δ18OH2O value for the water in equilibrium with wall rock is 1.6‰ (1000 lnαdol–H2O = 3.06 × 106/T2−3.24 after Zheng and Chen (2000), assuming that the temperature for the wall rock during nephrite formation was between 252° and 295°C). This value is lower than those of the fluids in equilibrium with most of the Xinjiang nephrite (figure 5). In addition, considering the characteristics of the chemical zoning (Liu et al., 2010), the higher δ18OH2O values for green nephrite fluids than for white ones in the Alamasi deposit (Wan et al., 2002) provide another indicator that oxygen isotopes decrease from granite to wall rock. However, the δ18OH2O value should have increased gradually if water unilaterally diffused from the granite to the wall rock, since water in equilibrium with nephrite is enriched or slightly depleted in 18O (depending on the temperature, calculated according to Equation 2 with T around 350°C). Considering that the δ2H value of the Alamasi nephrite is negatively related to the δ18O value (figure 4), the conflict can be explained by dualistic fluid sources. One is post-magmatic hydrothermal fluids provided by the granite forming the nephrite, while the other must be the meteoric water from the dolomite marble.
NEPHRITE-FORMING FLUIDS MODIFIED BY METAMORPHISM OR METASOMATISM
The hydrogen and oxygen isotopes of nephrites from Xiuyan (Duan and Wang, 2002; Wan et al., 2002; Wang et al., 2007) and Złoty Stok (Gil et al., 2015a) overlap with each other to some extent (figure 4). Their calculated fluid isotopes plot in the regional metamorphic water field (figure 5), which is in accordance with their geological environment. The Xiuyan nephrite occurs not far from the famed serpentine jade deposit formed from metamorphic hydrothermal fluids (Wu et al., 2014). Silicon isotope studies support the interpretation that the formation of the Xiuyan nephrite was related to metamorphic fluids (Duan and Wang, 2002; Wu et al., 2014). At Złoty Stok, some geological bodies related to serpentine occur not far from the dolomite-related nephrite deposit (Gil et al., 2015a,b).
Like the nephrite-forming fluids of Xiuyan and Złoty Stok, those of Dahua and Sanchakou plot in the metamorphic water field (figure 5). The δ18O values of the Dahua, Sanchakou, and Luodian nephrites are higher than others (with the exception of Tanghe), and these deposits are related to basic igneous rocks of diabase or gabbro (Zhou et al., 2006; Yang et al., 2012; Li et al., 2014; Zhang et al., 2015; Xu and Wang, 2016), which is distinct from other dolomite-related nephrites. The presence of siliceous components in the wall rocks is another common feature for these three deposits. The wall rock for Dahua nephrite is a suite of interbedded layers of calcirudite, calcarenite, and micrite mixed with laminar siliceous rocks and paramoudra (Xu and Wang, 2016). Yang et al. (2013) discussed the relationship between nephrite formation and siliceous veins in the Sanchakou deposit. The country rocks around the Luodian nephrite are siliceous clayey micrites and cherty limestones (Yang et al., 2012; Li et al., 2014). These silicalites compensate for the Si shortage during the formation of nephrite from basic rocks. For Luodian nephrite, this is supported by the δ18O equilibrium between quartz and nephrite. The δ18OQz value of the quartz from the deposit is 22.4‰ (Yang, 2013). Thus, the calculated δ18OTr value for tremolite by the quartz-tremolite fractionation equation 103lnQz–Tr = 2.25 × 106/T2 + 0.46 (Zheng, 1995) at 350°C equals 16.15‰, which is in the range of its nephrite δ18O value = 14.1‰–16.5‰ (Yang, 2013). The speculation of compensation is also supported by the Si isotope accordance between the nephrite and the siliceous veins, paramoudra, and silicalites (δ30Si = 1.1‰–1.7‰; Yang, 2013). These values, in combination with field observations, indicate that the hydrothermal fluid forming Luodian nephrite derived from either diabase intrusion (Yang et al., 2012; Zhang et al., 2015) or seawater circulation driven by diabase intrusion (Li et al., 2014). A comparable process occurred at Sanchakou: The water in the sediments convected with magmatic hydrothermal fluids (Zhou, 2006), or the acidic magmatic hydrothermal fluids that extracted Mg from gabbro (Yang et al., 2013) reacted with wall rocks and formed nephrite. Obviously, the hydrothermal fluids that formed these nephrites were no longer the original magmatic hydrothermal fluids, but rather the fluids that had been modified by metasomatism.
XINJIANG PLACER NEPHRITE ISOTOPES AND FLUID-ROCK REACTION
The Xinjiang placer nephrites, which are mainly dug out from paleo river beds flowing through the Takelamagan Desert, differ from the primary ones by their wide ranges of hydrogen and oxygen isotope ratios, especially δ2H (figure 4). There are four factors potentially influencing this difference:
- Impurities: Impurities may induce a conspicuously high δ2H value (Liu et al., 2016), as well as a wide range of variation.
- Compositional effect: Most of the placer nephrite tested featured high Fe (Liu et al., 2011b, 2016), which can result in a compositional effect on hydrogen isotope fractionations in a tremolite-H2O system (Vennemann and O’Neil, 1996).
- Complicated derivations: Since several primary deposits occur in the upper reaches of the Yulongkashi and Kalakashi Rivers, the placer nephrite might come from different primary deposits, even including serpentine-related nephrite (Liu et al., 2016).
- Fluid-rock reaction: The δ2H–δ18O trends of some of the Xinjiang placer nephrites are similar to those of the Alamasi nephrite (figure 4). The δ18O value, which is mainly controlled by the nephrite itself (Yui et al., 1990), has remained nearly constant after nephrite formation due to its high closure temperature of 424°C (Brady, 1995).
The closure temperature can be understood as the lowest temperature of isotope diffusion or loss. That is, the δ18O value of the placer nephrite is almost equal to that of the primary nephrites. The δ2H value of the placer nephrite, however, can be enhanced by the reaction between meteoric water (desert water that has been fractionated by evaporation; figure 5) and rock (nephrite).
The hydrogen in hydrous minerals diffuses rapidly and shows a closure temperature, below which it will no longer diffuse and change its composition, in cooling metamorphic rocks far below the formation temperature of the mineral assemblages (Graham, 1981). The closure temperature (Tc) for hydrogen isotope volume diffusion can be expressed as (Dodson, 1973):

where the time constant is

in which the activation energy for tremolite E = 71.5 kJ/mol (Graham et al., 1984; Farver, 2010); the gas constant R = 8.314 J/mol/K; the anisotropic factor for cylinder case A = 27 (Dodson, 1973); the pre-exponential factor in the Arrhenius relationship D0 = 1.21 × 10–8 m2/s, calculated from figure 5 of Graham et al. (1984). Thus, the closure temperature can be as low as 61°C (calculated by grain radius a = 0.5 μm, cooling rate dT/dt = –10°C/d) to 123°C (calculated by a = 1 μm, dT/dt = –50°C/d). Since the radius of nephrite tremolite can be smaller, the calculated closure temperature will decrease. Furthermore, an experiment showed that tremolite can dissolve at a pH of 6.9 at a low temperature of 37°C (Diedrich et al., 2014). Grapes and Sun (2010) suggested that higher porosity created by actinolite dissolution results in an exponential increase in weathering. Tremolite fibers, with lower iron concentration than actinolite, have high chemical reactivity as well (Pacella et al., 2015). Thus, the hydrogen isotope ratio can re-equilibrate at low temperature between the placer nephrite and meteoric water, enhancing the δ2H value of the former.
CONCLUSIONS
On the basis of formation environment and formation process, hydrogen and oxygen isotope ratios of nephrites from around the world can be analyzed. These isotope ratios, even for oxygen alone, appear to be discrimination criteria for the geographic origin determination of dolomite-related nephrites, especially those from Vitim (Russia), Chuncheon (South Korea) and the Xinjiang Uyghur Autonomous Region and Qinghai Province of China. However, the nephrite δ18O values from Xiuyan, Dahua, and Złoty Stok overlap. The isotopic ratio differences are mainly derived from the ore-forming fluids. The isotopes of dolomite-related nephrites from Russia, South Korea, Xinjiang, and Qinghai Province increase in sequence, and the ore-forming fluids vary in the order of meteoric water → mixture of magmatic water and meteoric water → mixed water that experienced metamorphism to some extent or is even dominated by metamorphic fluid. Furthermore, the hydrogen isotope of the placer nephrite from the Hetian region of Xinjiang could have been modified by meteoric water when it was buried in paleo river beds flowing through the desert.
Based on this limited data set, we show that isotope ratio analysis is a new gem origin identification tool for gemologists studying nephrite (similar to what other researchers have shown for emerald and corundum). However, we point out with caution that more data is needed to optimize our findings.




