D-Color Natural IIa Diamond with Walstromite Inclusion
Nondestructive testing methods such as Raman, photoluminescence (PL), and infrared spectroscopy have been widely applied to identify diamond and its inclusions. The NV0/− (nitrogen-vacancy) and V0 (vacancy) centers are used as a probe to confirm whether the color of a type IIa diamond is natural or caused by high-pressure, high temperature (HPHT) treatment or irradiation (Fall 2016 Lab Notes, pp. 299–301; D. Fisher et al., “The vacancy as a probe of the strain in type IIa diamonds,” Diamond and Related Materials, Vol. 15, No. 10, 2006, pp. 1636–1642).
Recently, the National Gemstone Testing Center (NGTC) laboratory in Shenzhen received a 3.0 ct diamond for identification. The stone was a standard round brilliant cut with D color, SI2 clarity, and a diameter of 9.2 mm. When exposed to DiamondView imaging, the diamond exhibited medium blue fluorescence. It was identified as type IIa because no nitrogen- or boron-related absorptions were detected in the 800–1400 cm–1 range and at 2802 cm–1. Nitrogen-vacancy centers NV0 (575 nm) and NV– (637 nm) were not detected, either.

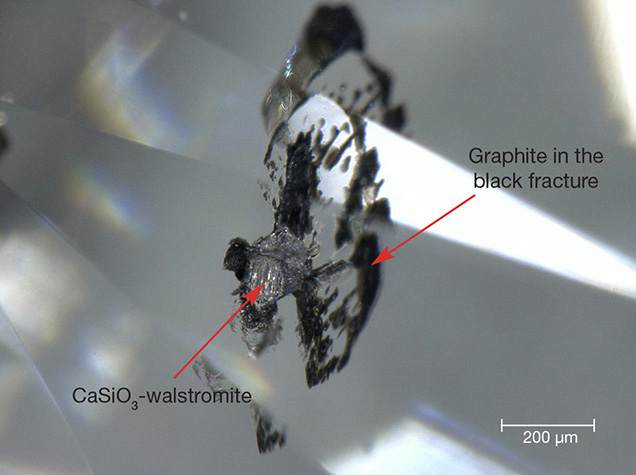
Magnification revealed some mineral inclusions and fan-shaped fractures (figure 1). Part of the fissure was filled with black material. We identified the inclusion in figure 1 as CaSiO3-walstromite and confirmed that the black fracture contained graphite using a Renishaw inVia micro-Raman confocal microscope equipped with a green solid laser (532 nm) focused through a 50× short-working-distance objective (figure 2). As the most abundant Ca-bearing mineral inclusion found in superdeep diamonds, CaSiO3-walstromite is believed to derive from CaSiO3-perovskite. Perovskite-structure minerals are predominant in the earth’s lower mantle more than 600 km below the surface. The presence of CaSiO3-walstromite and Ca-silicate inclusions is a strong indication of superdeep origin (C. Anzolini et al., “Depth of formation of superdeep diamonds: Raman barometry of CaSiO3-walstromite inclusions,” American Mineralogist, Vol. 103, No. 1, 2018, pp. 69–74).

The 3.0 ct diamond was similar to CLIPPIR (Cullinan-like, Large, Inclusion-Poor, Pure, Irregular, and Resorbed) diamonds (E.M. Smith et al., “Large gem diamonds from metallic liquid in Earth’s deep mantle,” Science, Vol. 354, No. 6318, pp. 1403–1405). Many CLIPPIR diamonds are of top color grades and type IIa, originating from a depth between 360 and 750 km. Dislocation networks, a relatively common feature in type IIa diamonds, were also found (figure 3). Dislocation networks in diamond are interpreted to be equivalent to the polygonized structure of dislocations in other minerals that have been deformed and subsequently annealed (H. Kanda et al., “Change in cathodoluminescence spectra and images of type II high-pressure synthetic diamond produced with high pressure and temperature treatment,” Diamond and Related Materials, Vol. 14, No. 11-12, 2005, pp. 1928–1931; K. De Corte et al., “Overview of dislocation networks in natural type IIa diamonds,” Fall 2006 G&G, pp. 122–123). High-temperature experimental treatment of synthetic type II diamond can also reproduce dislocation structures (H. Kanda et al., 2005; D. Fisher et al., “Brown colour in natural diamond and interaction between the brown related and other colour-inducing defects,” Journal of Physics: Condensed Matter, Vol. 21, No. 36, 2009, 364213).


CLIPPIR diamonds sometimes exhibit infrared absorption at 3107 cm–1; this is a nitrogen-bearing (VN3H) defect (J.P. Goss et al., “Identification of the structure of the 3107 cm–1 H-related defect in diamond,” Journal of Physics: Condensed Matter, Vol. 26, No. 14, 2014, 145801). This diamond has no clear signature of infrared absorption at 3107 cm–1 or NV centers (PL: 575 and 637 nm). We believe that the cause of this is an extremely low level of nitrogen, below the detection limits of the instrument (figures 4 and 5). The lack of nitrogen in type IIa diamond is associated with less color in the diamond. This diamond has a strong V0-GR1 center (741, 745 nm) and no NV0/− (575 nm) and V0 (637 nm) centers (figure 5).
The CaSiO3-walstromite inclusion in this 3.0 ct diamond indicates a natural diamond from a very deep origin.



