Metal Sulfide in Pyrope

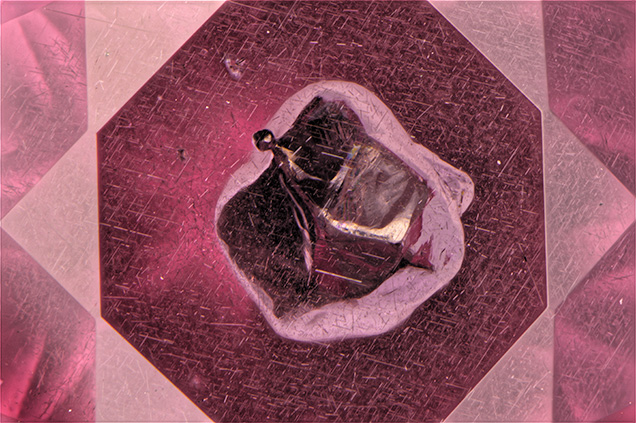
The 2.02 ct “inclusion gem” shown in figure 1 is not only spectacular to inclusion enthusiasts, but also a scientific mystery. This vanadium- and chromium-bearing pyrope was cut from one of the samples featured in G&G’s Winter 2015 issue (Z. Sun et al., “Vanadium- and chromium-bearing pink pyrope garnet: Characterization and quantitative colorimetric analysis,” pp. 348–369). The stone was faceted by Jason Doubrava (Poway, California) to display a relatively large, euhedral sulfide crystal inclusion. Sulfide crystals were observed in several of the samples in the 2015 study; the exact nature of the inclusions could not be determined by Raman analysis, but all displayed a metallic luster in surface-reflected light. Magnification also revealed a cloud of minute acicular inclusions resembling rutile surrounding the crystal (figure 2). Along with the elements expected for pyrope, such as Si, Al, Mg, Mn, and Fe, energy-dispersive X-ray fluorescence (EDXRF) analysis detected peaks for sulfur and rhodium. While sulfur would be expected in a metal sulfide, the rhodium peak is a mystery. Rhodium sulfide does exist, but explaining the presence of rhodium in this garnet would require destructive testing. In this case beauty trumps science, because this gem is truly a wonderful addition to the collection of this inclusionist and will thus remain a gemological curiosity.