Unusual Yellowish Green Spinel

Gem-quality spinel (MgAl2O4) occurs in a variety of colors based on the trace elements present within the stone. While synthetic spinels are available in almost any color, some colors are rarely found in natural spinel. The New York lab received a 2.54 ct light yellowish green spinel with unusually strong green fluorescence (figure 1). This variety of color, along with the strong fluorescence (in both long-wave and short-wave UV radiation) is rare in natural spinel, and we needed proof that this stone was not synthetic.
A refractive index of 1.715 suggested the stone might be natural (flame-fusion synthetic spinels typically have an RI of 1.728). Microscopic examination revealed a very small fingerprint shallow to the table facet. While not conclusively diagnostic for natural origin, it supported the possibility. When observed under cross-polarized filters, the stone revealed very little strain, more consistent with a natural spinel. To confirm natural origin, PL spectra and trace element chemistry data were collected.
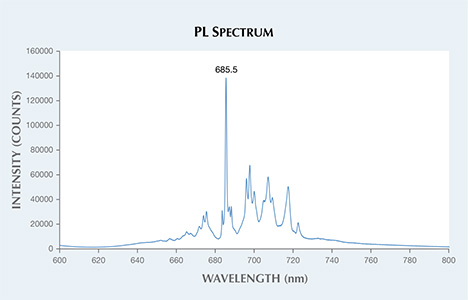
The PL spectra were collected at room temperature, using 514 nm laser excitation. The sharp and defined chromium emission features, with the strongest peak at approximately 685.5 nm (figure 2), verified that the stone was natural and unheated (S. Saeseaw et al., “Distinguishing heated spinels from unheated natural spinels and from synthetic spinels,” 2009). Heat treatment typically broadens and shifts the position of PL peaks (a similar effect is seen in synthetic spinels). Using laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS), high concentrations of natural trace elements were measured—particularly lithium, gallium, zinc, and beryllium. This reinforced our finding that the spinel was natural.
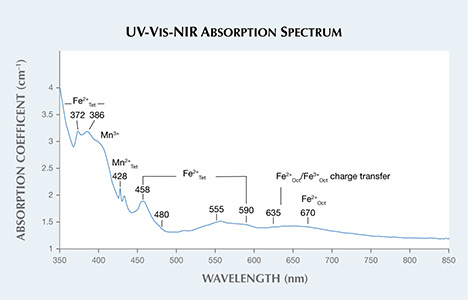
The stone also exhibited relatively high levels of manganese and iron. Fe can play various roles as a chromophore in spinel, depending on coordination within the crystal structure (divalent substitution of Mg and trivalent or divalent substitution of Al), but it is mostly responsible for different shades of blue and greenish blue (V. D’Ippolito et al., “Color mechanisms in spinels: cobalt and iron interplay for the blue color,” Physics and Chemistry of Materials, Vol. 42, 2015, pp. 431–439, http://dx.doi.org/10.1007/s00269-015-0734-0). Mn (divalent substitution of Mg and trivalent substitution of Al) is known to act as a yellow chromophore (among other colors) in spinels (F. Bosi et al., “Structural refinement and crystal chemistry of Mn-doped spinel: a case for tetrahedrally coordinated Mn3+ in an oxygen-based structure,” American Mineralogist, Vol. 92, 2007, pp. 27–33, http://dx.doi.org/10.2138/am.2007.2266). The combination of Fe and Mn within the crystal structure provided a transmission window in the green region of the visible spectrum (figure 3). Using a charge-coupled device (CCD) detector and a long-wave UV light source, the green fluorescence emission band was calculated to be centered at approximately 520 nm. This luminescence was attributed to Mn2+ cations (Summer 1991 Lab Notes, pp. 112–113). The fluorescence could have contributed to the overall color of the stone by adding more green hue through emission.
This was one of the most unusual colors of spinel examined by GIA.



