Optical Defects in Diamond:
A Quick Reference Chart
ABSTRACT
Gem diamonds owe much of their value to their color, or lack thereof. Defects in the atomic structure of diamond are responsible for this color and are important for the identification of color treatments. This article and its tables are intended as a quick reference for gemologists as they read about various common diamond defects in the gemological literature.
INTRODUCTION
Despite the commercial value of natural-color diamonds, distinguishing them from treated diamonds remains a significant identification challenge. While some diagnostic visual features exist (inclusions, color or growth zoning, and absorption bands seen with a spectroscope), the separation of natural from synthetic or treated diamonds is not always possible using standard gemological methods. In such cases, advanced spectroscopic analysis at a professional gem-testing laboratory is required. Imaging of luminescence distribution patterns is also a helpful tool for recognizing synthetic diamonds (Martineau et al., 2004; Shigley et al., 2004).
In a laboratory setting, the identification of diamonds is based mainly on the detection of tiny imperfections in the atomic lattice. These “defect centers” may include foreign impurity atoms (typically nitrogen, and occasionally boron or hydrogen); carbon atom vacancies in the lattice (either single or clusters of neighboring vacancies); carbon atoms positioned in between normal lattice locations (interstitials); and dislocations where planes of carbon atoms are offset from one another due to plastic deformation. Not all of these lattice imperfections create spectroscopic features, but several do so by allowing the diamond to absorb particular energies of incident light or radiation. Defects can occur randomly or in particular locations within the lattice. Diamonds can contain more than one type of defect, and in natural diamonds, defects can be altered over geologic time in the earth or by exposure to heat or radiation during color treatment.
So-called optical defects (or optical centers) cause absorption in the visible or near-visible portions of the electromagnetic spectrum, often producing coloration (e.g., figure 1). Luminescence reactions result when defects absorb higher-energy incident radiation and then reemit lower-energy radiation as visible light. Optical defects occur in very low concentrations in all diamonds, and their presence can be detected using spectroscopic techniques. A theoretically “pure and perfect” diamond containing no such defects would appear colorless.

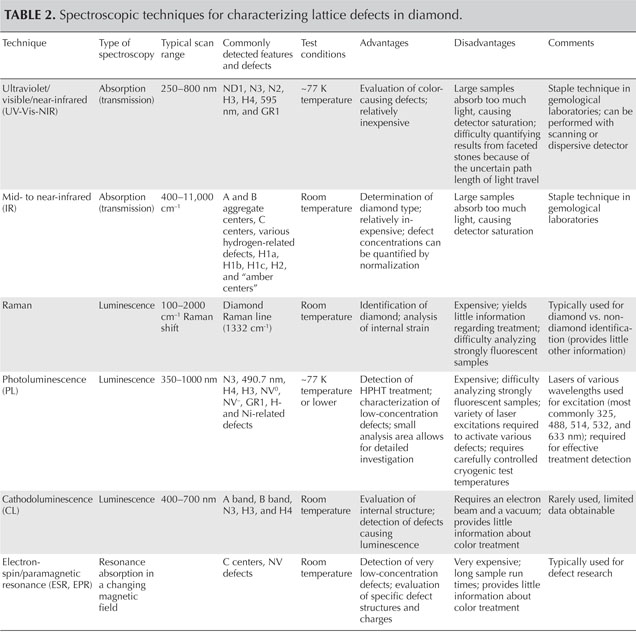
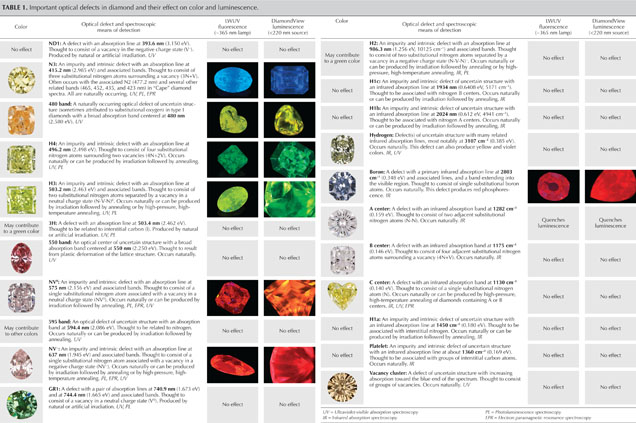
As a quick reference for gemologists, table 1 lists the most common lattice defects, including those that can create color and/or luminescence reactions in diamond. The scientific name of the defect, as well as the wavelength (or wavenumber) and electron volt (eV) positions of the main and associated spectral bands, are shown along with photos of representative diamonds. The most common spectroscopic technique used to detect the defect center is indicated by a code shown in italics.
Download a Larger Version of this Optical Defects Chart
Table 1. Important optical defects in diamond and their effect on color and luminescence.
Some precautions should be kept in mind when using this table. Each lattice defect is known to produce a particular diamond color or luminescence. For instance, the GR1 center produces green or blue-green color. But the reverse is not necessarily true—for example, not all green diamonds owe their color to the GR1 center. In fact, there are several causes of green color. In some cases, a diamond contains more than one optical defect, and its color stems from a combination of defects. In other cases, a diamond’s color may arise from absorption caused by one optical center, while its luminescence may result from another optical center.
Table 2 provides a comparison of the most common spectroscopic techniques for diamond characterization. For further reading on color in diamond, the Additional Reading list serves as a reference guide to access the much larger range of gemological and technical literature on diamond identification.