Nephrite Jade from Val Malenco, Italy: Review and Update
ABSTRACT
Alpe Mastabia, in the Val Malenco district of northern Italy, has been a source of nephrite jade since the early 2000s. Twenty-one samples from this locality were investigated by classical gemological methods; X-ray powder diffraction, combined with quantitative phase analysis; scanning electron microscopy in combination with energy-dispersive spectrometry; electron microprobe analysis; mass spectrometry; and mid-infrared spectroscopy. From a mineralogical standpoint, this jade consists mainly of tremolite amphibole, with variable amounts of other constituents, especially calcite (up to approximately 30 wt.%), but also pyroxene, apatite, and sulfide minerals. Its pale green color is related to the low iron content of the tremolite amphibole, whereas the other minerals are responsible for different colors (calcite for white, molybdenite and galena for gray). On the basis of minor and trace-element composition, we can classify this jade as dolomite-related nephrite (para-nephrite). Although new material could be recovered from this area, future production will probably be limited by access difficulties.

INTRODUCTION
Nephrite jade is an almost monomineralic rock, composed primarily of tremolite [Ca2Mg5Si8O22(OH)2] to actinolite [Ca2(Mg,Fe)5Si8O22(OH)2] amphiboles (Leake et al., 1997). Although tremolite-actinolite is considered the predominant phase in nephrite jade, its specific weight percentage range is still debatable.
Major sources include the Kunlun Mountains in Qinghai Province and the Xinjiang Uygur Autonomous Region of China; the East Sayan Mountains of Siberia; Chuncheon in South Korea; South Westland in the South Island of New Zealand; and Cowell, Australia (Harlow and Sorensen, 2005; Liu et al., 2011a,b; Zhang et al., 2011).
A new deposit of gem-quality nephrite jade (figure 1) was discovered at the beginning of the 2000s at Alpe Mastabia, located in the Val Malenco district in the Sondrio province of northern Italy (figure 2). Mr. Pietro Nana first noticed an attractive green stone in the discarded waste materials of an abandoned talc mine (figure 3) located at an altitude of 2,077 meters (Nichol and Geiss, 2005). The events leading to the discovery of the Alpe Mastabia nephrite as well as the geologic environment bear striking similarities to those reported by Dietrich and De Quervain (1968) for the better-known nephrite deposit at Scortaseo (Val Poschiavo, Switzerland), situated less than 20 km away.


This study aims to provide a review and update of the nephrite jade from Val Malenco, by investigating a suite of rough and cut gem-quality samples using X-ray powder diffraction combined with quantitative phase analysis based on the Rietveld method; scanning electron microscopy in combination with energy-dispersive spectrometry (SEM-EDS); electron microprobe analysis in wavelength dispersion mode (EMPA-WDS); mass spectrometry; and mid-infrared (IR) spectroscopy
GEOLOGIC SETTING
Val Malenco is an extremely interesting geological and mineralogical district (Adamo et al., 2009) situated in the Rhetic Alps near the Italian-Swiss border between the Southern Alps and the so-called “root zone” of the Alpine nappes. The main geological unit is an ultramafic body (the “Malenco unit”) that is one of the largest ophiolitic masses of the Alps (figure 2). It is exposed in an area of about 130 km2 and consists of serpentinized peridotite with minor relicts of lherzolite and harzburgite (Trommsdorff et al., 1993). The Alpe Mastabia talc mine is situated in a narrow tectonic zone (Lanzada-Scermendone) at the southern margin of the Malenco unit (figure 2). About 300 meters away is serpentinized rock, and the rocks around the talc mine are orthogneisses and schists (see box A) of the pre-Mesozoic crystalline basement, situated among Triassic white calcitic to dolomitic marbles. The origin of the talc deposit, as well as of tremolite and nephrite, is ascribed to metasomatic processes within the dolomitic marbles during the Alpine metamorphism (Montrasio, 1984; Nichol and Giess, 2005).
HISTORY AND PRODUCTION
During the years of operation at the Alpe Mastabia talc mine, from 1964 to 1994, nephrite boulders associated with the talc were ignored or discarded as waste (Andreis, 1970; De Michele et al., 2002; Nichol and Giess, 2005). The mine was abandoned, and entrances to its horizontal tunnels and galleries that once led to the ore body have mostly caved in or been barricaded (again, see figure 3), making the deposit difficult to access. In 1995, while examining the waste material outside the mine, Mr. Nana noticed some attractive nephrite boulders. Recognizing their gemological value, he consigned rough samples to lapidary workshops in China and Idar-Oberstein, Germany. The production and marketing of nephrite jade from Alpe Mastabia started at the beginning of the new millennium, with about 25 tons produced since the discovery. Production still flourishes due to the high quality of the finished jewelry pieces and other ornamental objects.
MATERIALS AND METHODS
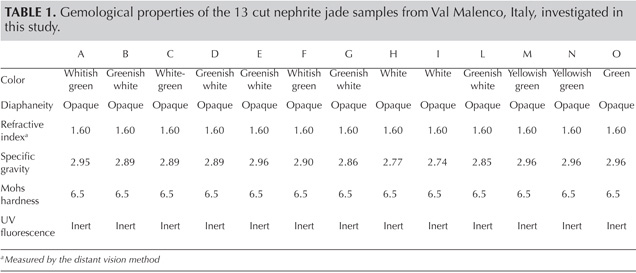
We examined 21 samples from the Val Malenco deposit, consisting of 13 cut (3.33–25.34 ct; figure 4) and eight rough (0.35–2.50 ct) specimens. All 13 cut samples underwent standard gemological testing to determine their refractive index (RI), specific gravity (SG), and ultraviolet (UV) fluorescence.

X-ray powder diffraction measurements were carried out on six rough specimens to determine the jade’s mineralogical composition. Data were collected by means of a Panalytical X’Pert-PROMPD X’Celerator X-ray powder diffractometer, using CuKα radiation (λ=1.518 Å) at a beam voltage of 40 kV and a current of 40 mA. X-ray powder diffraction patterns were collected over the 9–80° range of the scattering angle 2θ, with steps of 0.02° 2θ and a count time of 25 seconds per step. The phase identification was based on data from the PDF-2 database (International Center for Diffraction Data, Newton Square, Pennsylvania). Quantitative phase analysis (box B) was performed with the Rietveld method using the GSAS software package (Larson and Von Dreele, 1994) to treat the experimental 2θ-profile.
The microstructural features of six rough samples were investigated using a Cambridge STEREOSCAN 360 scanning electron microscope (SEM), with an acceleration current of 15 kV. Semi-quantitative chemical analyses were performed using the electron microscope’s EDS system (ISIS 300 Oxford).
We also performed quantitative chemical analyses in situ of the fibrous crystals constituting the four rough nephrite samples previously analyzed with X-ray powder diffraction. We used a JEOL JXA-8200 electron microprobe in wavelength-dispersive mode (EMPA-WDS) under the following conditions: 15 kV accelerating voltage, 15 nA beam current, and a count time of 60 seconds on peaks and 30 seconds on the background. The following standards were used: natural grossular (for Si and Ca), anorthite (Al), fayalite (Fe), olivine (Mg), rhodonite (Mn), omphacite (Na), ilmenite (Ti), K-feldspar (K), and pure V and Cr for those elements. The raw data were corrected for matrix effects using a conventional Φρ Z routine in the JEOL software package.
The trace-element composition of the same fibrous crystals in four rough samples was determined by laser ablation–inductively coupled plasma–mass spectroscopy (LA-ICP-MS). The instrument consisted of a Quantel Brilliant 266 nm Nd:YAG laser coupled to a Perkin Elmer DRCe quadrupole ICPMS. The spot size was 40 μm, using NIST SRM 610 glass as an external standard and Ca as an internal standard, as analyzed by microprobe. Precision and accuracy estimated on the basaltic glass standard BCR2 were better than 10%.
Additional information was derived from hydrogen isotope composition, obtained through multiple analyses of a few milligrams of selected tremolite fibers by mass spectrometry following standard procedures (Vennemann and O’Neil, 1993).
Mid-infrared absorption spectroscopy (4000–600 cm–1) was carried out on four rough specimens using a Nicolet Nexus Fourier-transform infrared (FTIR) spectrometer, operating in transmission mode with KBr pellets, at a resolution of 4 cm–1 and 200 scans per sample.
RESULTS
Gemological Properties. The gemological properties of the 13 cut jades from Val Malenco are listed in table 1.The color of both the polished and rough samples ranged from a common white and white-green to a rarer yellowish green and green (figure 4). Two rough samples had a blackish gray color (similar to the bracelet’s color in figure 11). The RI was constant, while SG ranged from 2.74 to 2.96, with the lower values in the whitest samples. The SG values of the two blackish gray rough samples were 2.80 and 2.86. All the stones were opaque and inert to long- and short-wave UV radiation. Irregular striped or spotted color zoning was observed in nearly every sample.
X-ray Powder Diffraction Data. Quantitative phase analysis based on XRPD data showed that five specimens consisted mainly of tremolite amphibole, with various amounts of other minerals, especially calcite. This mineral was generally less than 5 wt.%, though exceptional values of about 25–30 wt.% were found in the two most whitish samples (table 2; figure 5).


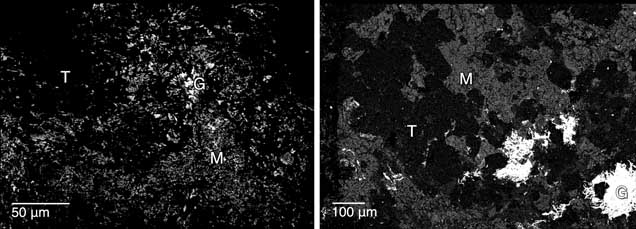
Microstructure. The nephrite jade from Val Malenco showed a micro- to crypto-crystalline texture that consisted of a dense intergrowth of fine (about 10–20 μm long) randomly oriented bundles of tremolite fibers (figure 6). Other minerals were identified by SEM-EDS as calcite, diopside, apatite, and opaque minerals (molybdenum, iron, lead, and zinc sulfides; see figure 7). Calcite was widespread in almost all samples, whereas the other minerals were rarer. In particular, molybdenite and galena were more concentrated in the blackish gray nephrite samples. These minerals were distributed unevenly in nephrite.

Chemical Composition. The chemical composition of the fibrous crystals in the jade is reported for four samples in table 3.
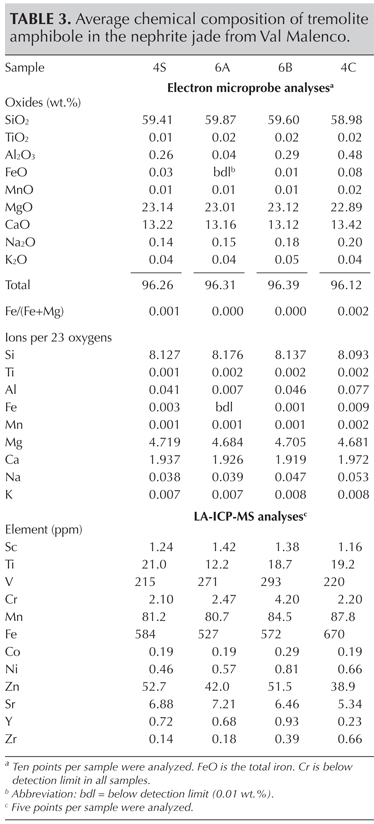
Chemical analyses of the fibrous mineral showed a composition close to that of tremolite amphibole, Ca2Mg5Si8O22(OH)2, according to the classification of Leake et al. (1997), with a low concentration of most trace elements. All elements of the first transition series (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn), as well as the alkaline earth metals (Sr, Ba), and alkaline metals (K, Rb, Cs) were always less than 0.1 wt.%, with the lone exception of Na, ranging from 0.14 to 0.20 wt.% as Na2O. Among the chromophore ions, iron was the most abundant, with contents ranging from 527 to 670 ppm (as Fe), followed by vanadium at about 200 ppm (as V); chromium was much lower, with concentration ≤4 ppm (as Cr; table 3). In terms of stable isotopic composition, the nephrite samples had a hydrogen isotopic mean value (δ D) of –113± 4.8‰.
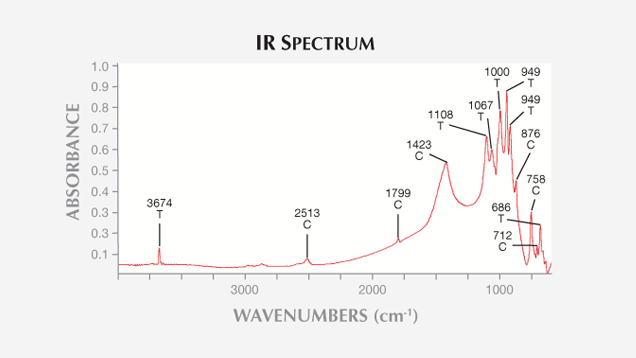
Mid-Infrared Spectroscopy. The samples’ mid-IR absorption spectra, collected in transmission mode with KBr pellets (figure 8), were characterized by bands at 3674, 1108, 1067, 1000, 949, 922, 758, and 688 cm–1 , typical of tremolite (Hawthorne and Della Ventura, 2007). The whitest samples (12 and 4C) also contained the absorption features of calcite (2513, 1799, 1423, 876, and 712 cm–1; Farmer, 1974).
DISCUSSION
The various samples from Val Malenco exhibited RI values typical for nephrite jade (O’Donoghue, 2006). Their SG values, which showed variation with the color of the stones, were in agreement with those commonly reported for this jade (SG=2.9–3.1; O’Donoghue, 2006), except for the lower values in the two white samples (2.77 and 2.74, respectively; see H and I, table 1). These SG values are compatible with a significant amount of calcite, whose SG (2.70 ± 0.01) is lower than that of tremolite. The Mohs hardness of 6.5 and the finely felted fibrous texture are typical of nephrite from localities worldwide (O’Donoghue, 2006).
The fine fibers of tremolite amphibole occurred together with other unevenly distributed constituents. Calcite was the most abundant, although its content varied from 0 to about 30 wt.%; pyroxene, apatite, and sulfide minerals were rarer. The variable amount of calcite corresponded with a white hue (figure 9), while molybdenite and galena were responsible for the gray and black hues.
Chemical analysis of the tremolite fibers revealed a low abundance of minor and trace elements, with iron as the most abundant chromophore ion. Yet its content shows comparable values in the various sample colors from white to green (again, see figure 9). This suggests that the occurrence of other mineral phases (e.g., calcite) could influence the jade’s color variation.

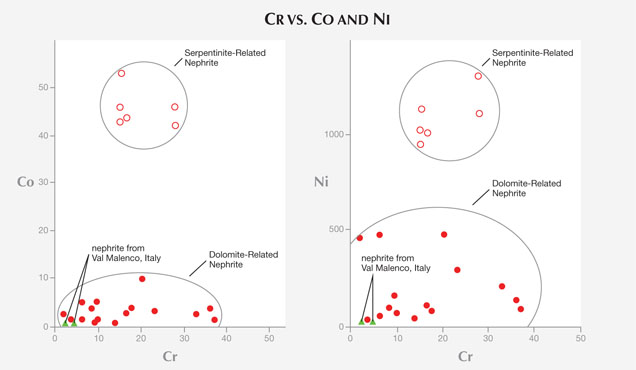
Nephrite’s minor and trace-element chemical composition plays an important role in tracing its geologic origin. It is used to distinguish nephrite associated with dolomitic marbles (dolomite-related serpentinite rock (serpentinite-related nephrite or ortho-nephrite); see, for instance, Nichol (2000) and Siqin et al. (2012). In particular, various authors (e.g., Siqin et al.) have shown that serpentine- and dolomite-related nephrite minerals can be separated on the basis of their Fe/(Fe+Mg) ratio (0.064–0.118 and 0.001–0.074, respectively) and their concentrations of Cr, Co, and Ni, which are higher in nephrite associated with serpentinite. Based on the very low Fe/(Fe+Mg) ratio (always 0.002), as well as the low contents of Co (0.19–0.29 ppm), Cr (2.10–4.20 ppm), and Ni (0.46–0.81 ppm) found in the nephrite jade from Val Malenco (table 3 and figure 10), we can classify this material as dolomite-related nephrite or paranephrite.
The possible formation process for this type of nephrite deposit is commonly ascribed to a metasomatic reaction involving dolomite replacement by silicic fluids (Harlow and Sorensen, 2005). This is consistent with the reaction proposed by Nichol and Giess (2005) for nephrite from Val Malenco, which produces excess calcite as isolated grains or aggregates:
5CaMg(CO3)2 + 8SiO2 + H2O = Ca2Mg5Si8O22(OH)2 + 3CaCO3
(dolomite) (silica) (tremolite) (calcite)
Yet the occurrence of diopside grains as rare relicts within tremolite also suggests a second stage of formation involving the intermediate formation of diopside from dolomite, subsequently replaced by tremolite. In any case, the formation of the Alpe Mastabia deposit in Val Malenco implies an intense fluid-rock reaction by hydrothermal solutions, percolating at the contact between the dolomitic marbles and the enclosing schists (Nichol and Giess, 2005). The very low δD-depleted value determined on this nephrite also suggests a possible contamination of the hydrothermal fluid with water of meteoric origin (Yui and Kwon, 2002; Harlow and Sorensen, 2005).
CONCLUSIONS
Nephrite jade from Val Malenco, northern Italy, entered the market at the beginning of the 2000s. Since the discovery of nephrite there in 1995, the deposit has produced about 25 tons of gem-quality rough, carved into ornamental objects or fashioned into jewelry (figure 11). This study, performed on both cut and rough specimens, shows that nephrite jade from Val Malenco is composed of tremolite amphibole. It contains a low concentration of iron as a chromophore, in agreement with its pale green color. Variable amounts of other constituents also influence the color: Calcite is responsible for the rather common white hue, while molybdenite and galena cause the black and gray colors. The nephrite’s compact microstructure and soft color make it an attractive gem material. Although new finds could be limited by access difficulties, the geologic features of the deposit suggest significant potential for further production.

| Box A: Geological Glossary |
|
Nappe: A large body or sheet of rock that has shifted far from its original position by thrust faulting during continental collisions. Also known as an allochton. Ultramafic (or ultrabasic): Describing igneous and meta-igneous rocks with very low silica content (less than 45% SiO2), generally greater than 18% MgO, high FeO, and low potassium. Ultramafic rocks are usually composed of greater than 90% mafic minerals, which are dark and have high magnesium and iron contents. Peridotite: Coarse-grained ultramafic rock consisting mainly of olivine (at least 40% by volume) and pyroxene (ortho-/clino-). Peridotites are distinguished according to their different mineralogical composition. Dunite contains more than 90% olivine. Wherlite is mostly composed of olivine (40–90%), clinopyroxene, and a minor amount of orthopyroxene (10%). Lherzolite contains olivine (40–90%) and approximately equal amounts of clinopyroxene and orthopyroxene. Harzburgite contains olivine (40–90%), orthopyroxene, and small amounts of clinopyroxene (10%). Orthogneiss: Rock derived from the metamorphism of igneous rocks. Orthogneiss is distinguished from paragneiss, which derives from sedimentary rocks. Schist: Metamorphic rock having a foliated or plated structure called schistosity, in which the flaky minerals (micas) are visible to the eye. Metasomatic: Referring to a process by which the chemical composition of a rock or portion of rock is altered in a pervasive manner. Metasomatism involves the introduction or removal of chemical components as a result of the rock’s interaction with aqueous solutions. During metasomatism, the rock remains in a solid state. |
| Box B: Quantitative Phase Analysis |
| Quantitative phase analysis (QPA) is used to determine the concentration of various phases present in a mixture after the identity of every phase has been established (qualitative phase analysis). Powder diffraction is a direct method to identify and quantify phases on the basis of their unique crystal structures (Pecharsky and Zavalij, 2003; Dinnebier and Billinge, 2008). Among existing QPA methods, the Rietveld (1969) technique appears to be one of the fastest and most reliable. The foundation of the Rietveld method is that the difference between the measured and calculated whole powder diffraction profile should be close to zero by means of the following minimized function (Pecharsky and Zavalij, 2003): Φ = Σni=1 wi (Yiobs – Yicalc)2 where Y1 obs is the observed and Y1 calc is the calculated intensity of a point i of the powder diffraction pattern, and wi is the weight assigned to the ith data point. The entire calculated powder diffraction pattern is based on simultaneously refined models of the crystal structures, diffraction optics effects, instrumental factors, and other specimen features. Rietveld refinement of multiphase samples can generate a relatively accurate QPA, because the Rietveld scale factors determined during the refinements for every phase in the mixture are proportional to the weight of the corresponding phases (Pecharsky and Zavalij, 2003; Dinnebier and Billinge, 2008). |



