Pink Pyrope Garnet
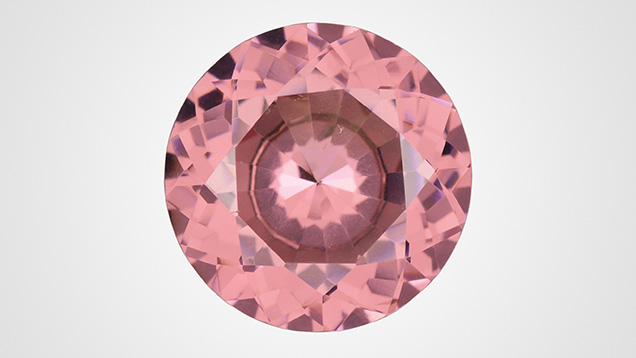
The Carlsbad laboratory received a 7.26 ct pink modified round brilliant (see above) for a colored stone identification report. Standard gemological testing revealed that the stone was singly refractive with a refractive index (RI) of 1.741 and a hydrostatic specific gravity (SG) of 3.77, properties consistent with garnet. Internally the stone was quite clean, with microscopic examination revealing scattered short needles.
The garnet group is composed of more than 20 species, all of them sharing the basic chemical formula X3Y2(SiO4)3. Five of these species are common within the jewelry industry: almandine, andradite, grossular, pyrope, and spessartine. Andradite and grossular are ugrandite garnets; they have calcium in the X site of their chemical formula. Almandine, pyrope, and spessartine are all pyralspite garnets containing aluminum in the Y site. Isomorphous replacement, in which one chemical element substitutes for another in a mineral’s crystal structure, makes it possible for garnets to be a chemical mixture of two or more garnet species. GIA gemologists use a garnet’s gemological properties and chemistry to categorize the stone into its particular species.
Chemical analysis revealed that the 7.26 ct stone was a pyralspite garnet due to the high aluminum concentration (values expressed in wt.%: MgO 18.50%, Al2O3 23.05%, SiO2 41.32%, CaO 1.89%, TiO2 0.05%, V2O3 0.02%, Cr2O3 0.04%, MnO 11.93%, Fe2O3 3.17%). The composition of this garnet and its gemological properties were consistent with pyrope, which has an RI of 1.73–1.75, an SG of 3.78 (+0.009/–0.016), and magnesium dominating the X site and minor amounts of manganese and iron. Pyrope garnet, though, has a color range of red to reddish orange and colorless. Pink pyrope garnet is incredibly rare, especially in stones of this size.
Pyrope-spessartine can have a bodycolor similar to pyrope but has an RI range of 1.75 to over the limit and an SG of 3.78, both higher than the values documented for this pink garnet. The fact that garnet can be a mixture of species can make the identification of these stones challenging. This example is an important reminder to carefully analyze a garnet’s gemological properties to accurately identify it and how the laboratory can use chemical analyses as validation.



