HPHT-Grown Colorless Diamond Displaying Unusual Phosphorescence Effects
Phosphorescence longer than 10 seconds in reaction to ultraviolet radiation is regularly observed in colorless to near-colorless and blue-colored diamonds grown by a high-pressure, high-temperature (HPHT) technique. The gemological laboratory of Stuller Inc. recently tested an HPHT-grown colorless (approximately E color grade with a blue-gray overtone) oval brilliant diamond weighing 0.71 ct that showed unusual fluorescence and phosphorescence reactions.
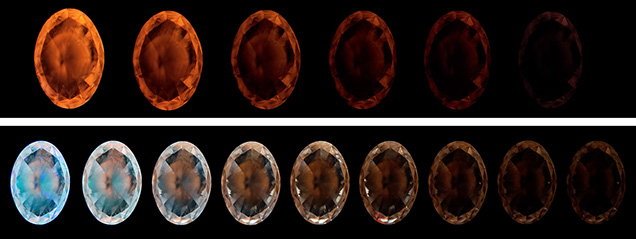
When exposed to a long-wave ultraviolet (LWUV, 365 nm) source for 60 seconds using a standard UV viewing cabinet, the stone emitted a gradually increasing strong orange fluorescence, followed by a long-lasting noticeable phosphorescence (~150 seconds, figure 1, top). The fluorescence and phosphorescence reactions demonstrated a nonuniform color distribution. Similar results were observed using other light sources, including 395 nm UV LED, 405 nm blue laser, a tungsten incandescent flashlight, and a fiber-optic halogen unit.
Given that orange phosphorescence can also be generated by low- and non-UV light sources, further analysis was performed using a series of spectral bandpass filters to identify other wavelengths that stimulate the orange phosphorescence. Testing demonstrated that medium- to strong-intensity light centered at around 430 nm produces the excitation.
Under short-wave ultraviolet (SWUV, 254 nm) radiation using a standard viewing UV cabinet and a De Beers PhosView instrument, the stone displayed a mix of strong greenish blue and medium orange zoned fluorescence and phosphorescence (figure 1, bottom). The colored phosphorescing areas decayed at different rates, with the greenish blue fading faster than the orange (~90 seconds vs. ~240 seconds, with a half-life of about 25 and 35 seconds, respectively).

Deep-UV (<225 nm) imaging demonstrated a strong greenish blue fluorescence with a clear cuboctahedral structure associated with HPHT growth (figure 2). After removal from the excitation source, the stone displayed a phosphorescence reaction similar to the reaction under SWUV.

Infrared spectroscopy revealed a type IIb diamond with relatively high levels of electrically uncompensated boron at 4089, 2929, 2800, and 2455 cm–1 (figure 3). Photoluminescence spectroscopy with 532 nm laser excitation at liquid nitrogen temperature (77K) showed no results, except for the diamond’s first- and second-order Raman spectrum peaks, a common tendency in HPHT-grown colorless diamonds (S. Eaton-Magaña et al., “Observations on HPHT-grown synthetic diamonds: A review,” Fall 2017 G&G, pp. 262–284).
The two phosphorescence colors emitted and their relatively long duration are well-known properties of HPHT-grown diamonds (S. Eaton-Magaña and R. Lu, “Phosphorescence in type IIb diamonds,” Diamond and Related Materials, Vol. 20, No. 7, 2011, pp. 983–989; Eaton-Magaña et al., 2017), and a blue-orange luminescence combination in one gem was also previously reported (Eaton-Magaña and Lu., 2011; B. Deljanin et al., “NDT breaking the 10 carat barrier: World record faceted and gem-quality synthetic diamonds investigated,” Contributions to Gemology, Vol. 15, No. 1, 2015) but of weaker intensity. The laboratory-grown diamond’s property of bicolor, decay-varying phosphorescence has been previously reported as well (K. Watanabe et al., “Phosphorescence in high-pressure synthetic diamond,” Diamond and Related Materials, Vol. 6, No. 1, 1997, pp. 99–106; Eaton-Magaña and Lu, 2011; Ulrika F.S. D’Haenens-Johansson et al., “Large colorless HPHT-grown synthetic gem diamonds from New Diamond Technology, Russia,” Fall 2015 G&G, pp. 260–279).
Considering the HPHT growth method, the long-lasting greenish blue phosphorescence produced by exposure to SWUV and deep UV was expected. It was previously suggested to be related to nitrogen-boron donor-acceptor pair recombination (Watanabe et al., 1997). However, the orange phosphorescence demonstrated a new stimulation source. Its combination with the greenish blue phosphorescence and the identified visible-light excitation wavelength reinforces the theory that the recorded orange phosphorescence is of a different donor-acceptor pairing.
Light of 430 nm wavelength can be found in most fluorescent and all incandescent indoor light sources, as well as the desk lamps and color grading cabinets found in gemological laboratories. Therefore, considering the possibility of more diamonds with this property in the market, their phosphorescence produced from visible light makes it challenging to assign a color in the colorless to near-colorless range for these diamonds during grading. Even under standard non- or low-UV output 6500K fluorescent bulbs, the orange luminescence generated by the source becomes noticeable, adding a yellowish overtone to the blue-gray bodycolor and changing its apparent color grade to approximately F to G. Such a change may impair the diamond grader’s ability to accurately assess the stone’s bodycolor.



