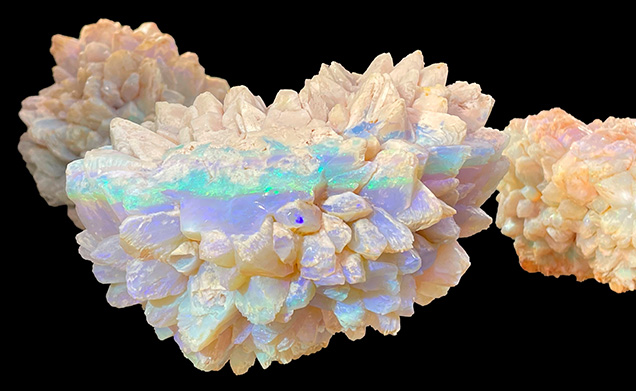
Rare Double Pseudomorph Ikaite-Calcite-Opal

At the Pueblo Gem & Mineral Show, Graeme Dowton of Red Earth Opal (White Cliffs, Australia) exhibited rare double pseudomorph ikaite-calcite-opal gem specimens mined from the Naatji Nest mine at White Cliffs in New South Wales, Australia. Ikaite was initially substituted with calcite and then opalized, therefore making these double pseudomorph opals. Also called “pineapple opal” in the market due to the form of their clusters, they are found exclusively in White Cliffs and typically occur within weathering-bleached siltstones and claystones. According to Dowton, no other type of gem-quality opal was found in the same layer where the pineapple opals were mined. During mining, it was noticed that two opals had formed within a few feet of each other but with distinctly different coloration: One displayed a rich spectrum of play-of-color, while the other showed almost no coloration. The 3,510 ct Heart of Australia (figure 1) shows rich play-of-color and is considered one of the finest specimens of this type ever unearthed. In some of Dowton’s samples, the precursor calcite was not fully substituted by silica gel, and white calcite crystals can be observed (figure 2).
Ikaite, CaCO3•6H2O, is a rare and metastable hydrated carbonate in sedimentary rocks that has only been identified in environments ranging from –2° to 7°C in nature (M.L. Vickers et al., “The ikaite to calcite transformation: Implications for paleoclimate studies,” Geochimica et Cosmochimica Acta, Vol. 334, 2022, pp. 201–216). The occurrence of ikaite suggests a period of very cold to near-freezing paleoclimate conditions in White Cliffs. At ambient temperatures (10°–30°C), ikaite transforms to more stable carbonate polymorphs such as calcite, aragonite, and/or vaterite. The ikaite and its following pseudomorphs act as paleothermometers (D. Shearman and A. Smith, “Ikaite, the parent mineral of jarrowite-type pseudomorphs,” Proceedings of the Geologists Association, Vol. 96, No. 4, 1985, pp. 305–314). When the host sedimentary rocks were weathered, they released silica into groundwater. This silica-bearing groundwater contacted with calcite, which gradually dissolved and reprecipitated to opal over time (B. Pewkliang et al., “The formation of precious opal: Clues from the opalization of bone,” Canadian Mineralogist, Vol. 46, No. 1, 2008, pp. 139–149). However, the detailed formation mechanism of ikaite-calcite-opal in White Cliffs requires future investigation.



