Chrysoberyl from the New England Placer Deposits, New South Wales, Australia
ABSTRACT
Mineralogical, chemical, and spectroscopic properties of chrysoberyl crystals recovered from sapphire placer deposits, related to Tertiary volcanic rocks, in the New England gem fields in New South Wales (NSW), Australia, are presented. The samples appeared yellow, yellowish brown, or brown in transmitted light, and some crystals revealed a distinct sectorial zoning between brown i (011) and yellow o (111) growth areas. In reflected light, the i sectors showed a whitish appearance, and cabochon-cut samples with larger whitish i sectors displayed chatoyancy. On the basis of morphological properties, trace-element contents, and absorption spectra, the chrysoberyl samples were subdivided into four different groups, possibly originating from different host rocks. The largest such group, comprised of samples with distinct sectorial color zoning, also revealed a pronounced variation in trace-element levels of titanium, niobium, and tantalum between the different growth sectors. Smaller variations were found for boron, magnesium, and iron, and almost no variation was observed for gallium.
INTRODUCTION
Chrysoberyl is formed through various magmatic and metamorphic processes. Two broad categories of deposits are widely known: those related to pegmatitic activity and those related to high-grade metamorphism. In particular, chrysoberyl is frequently crystallized directly from a pegmatite melt or in a reaction zone between a pegmatitic melt and aluminum-rich host rocks. With respect specifically to the chromium-bearing color-change chrysoberyl variety alexandrite, formation often occurs by reaction of a pegmatite intruding mafic or ultramafic rocks. Chrysoberyl is also found in high-grade (amphibolite-facies or granulite-facies) metamorphic rocks. Augmenting these primary occurrences, placer deposits may be derived from any of the foregoing types (Okrusch, 1971; Soman and Druzhinin, 1987; Franz and Morteani, 2002; Černý, 2002; Barton and Young, 2002; Beurlen et al., 2013). Such secondary deposits of gem-quality chrysoberyl related to high-grade metamorphic rocks are found, for example, in Sri Lanka, India, Tanzania, and Madagascar (Menon et al., 1994; Gunaratne and Dissanayake, 1995; Henn and Milisenda, 1997; Dissanayake et al., 2000; Milisenda et al., 2001; Manimaran et al., 2007). Likewise, secondary deposits related to pegmatites or pegmatites intruding aluminum-rich rocks have been discovered, for instance, in various Brazilian states (Proctor, 1988; Cassedanne and Roditi, 1993; Pedrosa-Soares et al., 2009).
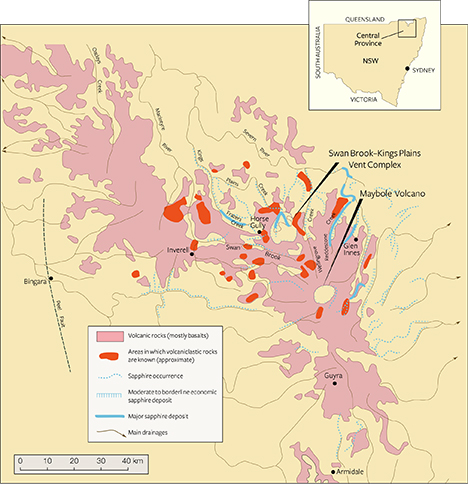
Chrysoberyl recovered with sapphires related to volcanic host rocks, in contrast, is extremely rare. Among the limited discoveries, this type of chrysoberyl has been mentioned in connection with secondary deposits in Australia, related to Tertiary volcanic host rocks, including in Anakie, Queensland (Brightman, 1984); in the New England gem fields of New South Wales (NSW; figure 1) (Coenraads, 1990, 1991, 1995); and in northeastern Tasmania (Sweeney, 1995; Bottrill, 1996). Further mineralogical or gemological information, however, is minimal. For instance, although chrysoberyl was recognized in the late 19th century as occurring in association with gem-quality sapphires in the secondary New England gem fields (Liversidge, 1876, 1888), no comprehensive description of the material is available. Thus, the present study was undertaken to examine a collection of samples from these New England placer deposits and thereby to determine mineralogical and chemical properties for this type of chrysoberyl.
MATERIALS AND METHODS
Because chrysoberyl associated with Australian sapphires shedding from Tertiary basalts and pyroclastics occurs only very rarely, miners often do not recognize the crystals. Rather, the stones are mistaken for corundum and sold within parcels of yellow and parti-colored rough sapphires.
The present study began with 39 crystals or crystal fragments and one chatoyant cabochon previously cut from such material. The research material was selected by one of the authors (TSC) from approximately 1 kg of rough, which had been purchased from the late Tom Nunan, one of the larger sapphire miners operating in the New England sapphire fields (Coldham, 2014). The rough parcel was comprised of a collection of atypical-appearing stones that were set aside by sapphire sorters over many years from the production of several mines in the New England region, including Swanbrook Creek, Reddestone Creek, and Kings Plains (for a general overview of the New England sapphire fields, see Coenraads, 1990, 1991, 1994; Abduriyim et al., 2012 a,b). These unusual stones were essentially anything that had caught the eyes of the sorters by virtue of being different from the blue, yellow, green, and low-quality sapphire commonly seen (figure 2). The collection included multiple types of material rare to the area, such as pink, purple, red, and orange corundum; unusually shaped stones; and those with strange color banding. Most stones within this kilogram of rough material were quite small, under 2 ct in weight.

As might be expected from the process of visual selection just described, the possibility remained that the initial 40 research samples (in total weighing about 65 carats) might still contain some corundum crystals. For this reason, all rough samples were first examined by traditional gemological methods, especially in the immersion microscope to facilitate observation of specific growth structures and sectorial zoning. Because some of the smaller crystal fragments did not show any microscopic properties of diagnostic value (i.e., neither characteristic growth structures nor mineral inclusions), these smaller samples were tested by micro-Raman spectroscopy using a Horiba XploRA confocal Raman microscope facility with a 532 nm laser. Micro-Raman spectroscopy was also employed to confirm the identity of several larger crystals. In total, 20 samples were examined by Raman spectroscopy; ultimately, six smaller crystal fragments were identified as yellow corundum. The present study is thus based on the remaining 34 chrysoberyl samples originating from the secondary New England sapphire deposits. To have found and selected only this small quantity of chrysoberyl associated with many thousands of kilograms of rough sapphire related to Tertiary volcanics mined over many years reiterated the rarity of the material.
The weight and size of the research material ranged from 11.71 ct (17.4 × 8.9 mm) to 0.45 ct (5.3 × 2.9 mm) for the rough samples. Coenraads (1995) reported similar sizes in the 10 to 5 mm range for chrysoberyl from the New England gem fields.
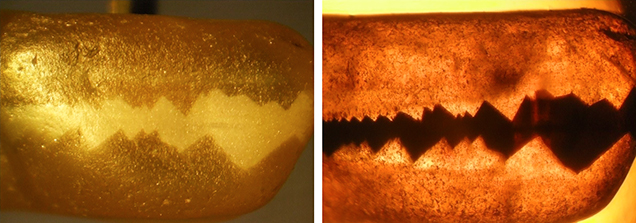
To better observe the structures without interference from the rough, heavily corroded and/or mechanically abraded or otherwise contaminated surfaces, certain samples were “windowed” with one or two polished faces, and a small group of transparent stones was completely faceted (figure 3, left). A few samples with larger whitish areas were cut as cabochons showing chatoyancy (figure 3, right).

In the present paper, the term “sectorial zoning” or “sectorial color zoning” is used to describe a different coloration between adjacent growth sectors, while “color zoning” refers to different colors within a specific growth sector. For all the chrysoberyls studied (samples in the as-received state, windowed crystals, or cut samples), growth structures, sectorial zoning, and color zoning were determined by immersion microscopy in methylene iodide using the methods described by Schmetzer (2011). Four cabochon-cut samples showing chatoyancy were examined in reflected light using a Leitz Ortholux II Pol-BK polarization microscope at high magnification (up to 1000×).
To obtain an overview of the qualitative chemical composition, 10 chrysoberyls were tested by energy-dispersive X-ray fluorescence spectroscopy (EDXRF) using a Bruker Tracer III-SD handheld unit.
Quantitative trace-element composition of 12 chrysoberyls was determined by means of laser ablation–inductively coupled plasma–mass spectroscopy (LA-ICP-MS), employing a Quantel Brilliant 266 nm Nd:YAG laser coupled to a PerkinElmer DRCe quadrupole ICP-MS. NIST SRM 610 glass was used as the external calibration standard, and Al served as the internal standard. The spot size was set to approximately 50 µm and the frequency to 10 Hz. To determine chemical zoning within the samples, traverses consisting of four to twelve single analysis points were recorded for all 12 chrysoberyls examined by LA-ICP-MS. The analysis was carried out on 55 elements; only those elements with concentrations higher than the detection limits are reported (see table 3).
Absorption spectra were obtained for six of the chemically analyzed samples with a CCD-type Czerny-Turner spectrometer in combination with an integrating sphere (for further details, see Schmetzer et al., 2013a). Only non-polarized spectra were recorded.
RESULTS
The chrysoberyls from the New England placer deposits in New South Wales were free of mineral inclusions when examined using the magnification of the gemological microscope (up to 100×), but they showed variation in chemical composition, internal morphology (growth structures and sectorial zoning), color, and color zoning. Thus, trace-element contents, color, internal growth features, sectorial zoning, color zoning, and spectroscopic properties were used to subdivide the samples into four primary groups, designated as groups I through IV in this study (figure 4). The few samples from the original group of 40 that showed mineral inclusions in the gemological microscope (e.g., zircon crystals with tension cracks) were all identified by Raman spectroscopy as corundum.

Morphology and Growth Features. The different crystal forms present in the New England chrysoberyls are listed in table 1. The habit of the samples was formed by the combination of two pinacoids a and b; three prism faces i, s, and r; and three dipyramids o, w, and n.
| TABLE 1. Morphology of chrysoberyls from the New England sapphire fields, NSW, Australia. | |||
| Crystal form | Designation | Miller indices (hkl)* | |
| Pinacoid | a | {100} | |
| b | {010} | ||
| Prism | i | {011} | |
| s | {120} | ||
| r | {130} | ||
| Dipyramid | o | {111} | |
| w | {122} | ||
| n | {121} | ||
|
*Based on a morphological cell with a = 4.42, b = 9.39, c = 5.47
|
|||
| Characteristic views | |||
| Direction of view | Symbol | Faces observed | |
| Parallel to the a-axis | [100] | b, i | |
| Parallel to the c-axis | [001] | a, b, s, r | |
| Intermediate between band c-axes (parallel to the prism i and the dipyramid o) |
[011] | i, w, o, a | |
| Intermediate between a-, b-, and c-axes |
[111] | i, n | |
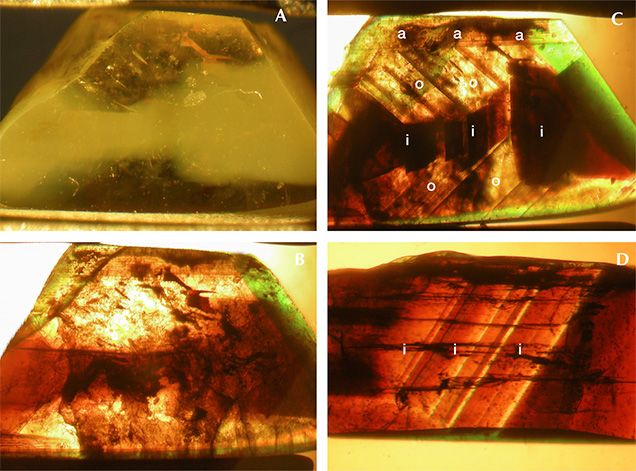
When examined in the immersion microscope, there were three main directions of view presenting the major internal growth features: parallel to the a-axis, parallel to the c-axis, and intermediate between the b- and c-axes. A fourth direction intermediate between the a-, b-, and c-axes was of less importance (see also table 1). A characteristic crystal showing the main crystal forms is depicted in figure 5A. In a view parallel to the a-axis, growth features parallel to the prism i and occasionally parallel to the pinacoid b were observed (figures 5C and 6). In a view parallel to the c-axis, growth features and faces seen were the pinacoids a and b, the prism s, and less frequently the prism r (figures 5B and 7). The different growth sectors in this latter view could also show sectorial zoning and color zoning.
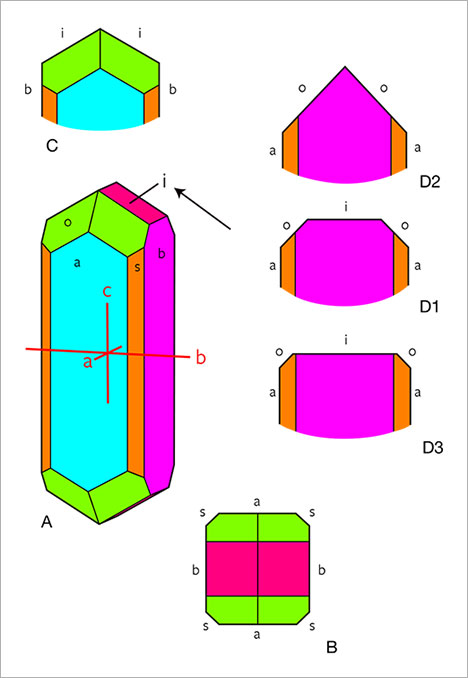

In a view between the b- and c-axes and parallel to the [011] direction (i.e., parallel to the prism i and the dipyramid o), the faces a, o, and i were observed, occasionally in combination with a small w dipyramid. This view also revealed the principal variation among the samples in crystal morphology (figure 5, D1, D2, and D3). In some samples, the size of the i prism faces was balanced with the size of the o dipyramids (figure 5, D1). In others, either the o or the i faces predominated. If the o faces were dominant, the i prism was small or not observed (figure 5, D2). If the i faces were dominant, the o dipyramid was smaller (figure 5, D3).
In an intermediate direction between the a-, b-, and c-axes, a combination of i and n faces was occasionally seen (figure 14).
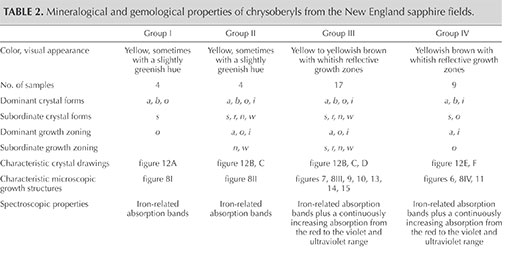
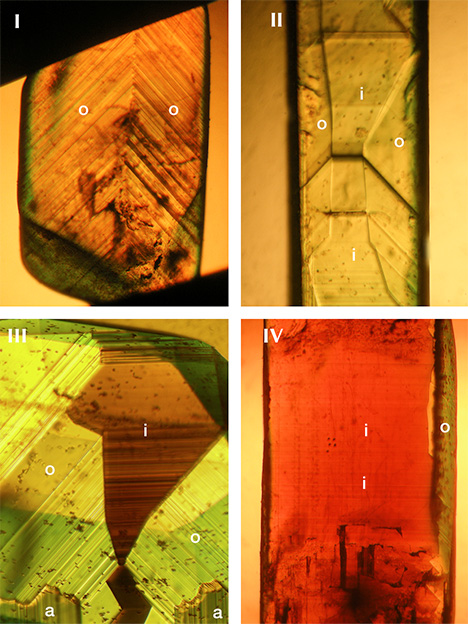
Taking into account color and these just-described morphological features, the four groups were characterized as follows (see table 2 and figure 8, examples I through IV):
- Group I: yellow color, o dipyramids dominant, no sectorial zoning, no color zoning
- Group II: yellow color, size of o dipyramids and i prism faces balanced, weak to absent color zoning or sectorial zoning
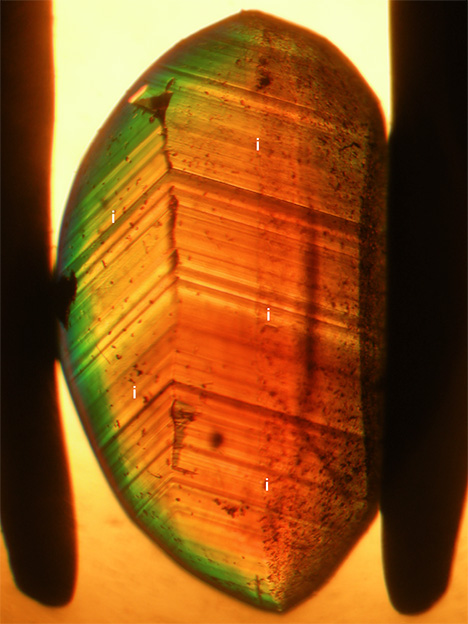
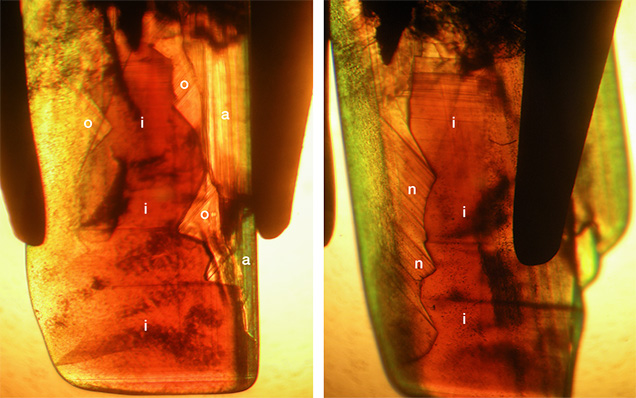
- Group III: yellow to brownish yellow or yellowish brown color, size of o dipyramids and i prism faces balanced, strong sectorial zoning in which o growth sectors were yellow and i growth sectors had a whitish appearance in reflected light but were yellowish brown to brown in transmitted light, color zoning in various zones but primarily in i growth sectors (see also figures 9 and 10)
- Group IV: brownish yellow or yellowish brown color, i prism faces dominant, sectorial zoning in which small o growth sectors were yellow and i growth sectors were whitish in reflected light but yellowish brown to brown in transmitted light, color zoning mainly in i growth sectors. This group also contained the only two twinned crystals within the 34 chrysoberyls examined (figure 11).
The samples of groups I and II and the predominantly yellow zones of chrysoberyls from groups III and IV occasionally showed an additional slight greenish hue.
The second morphological feature that influenced the habit of the crystals was the relative size of the a and b pinacoids. Chrysoberyls in which the sizes of the a and b pinacoids were balanced showed prismatic habit (figure 12 A,B,D,E), while samples with larger a faces were platy or tabular (figure 12C) and could also be twinned (figure 12F).
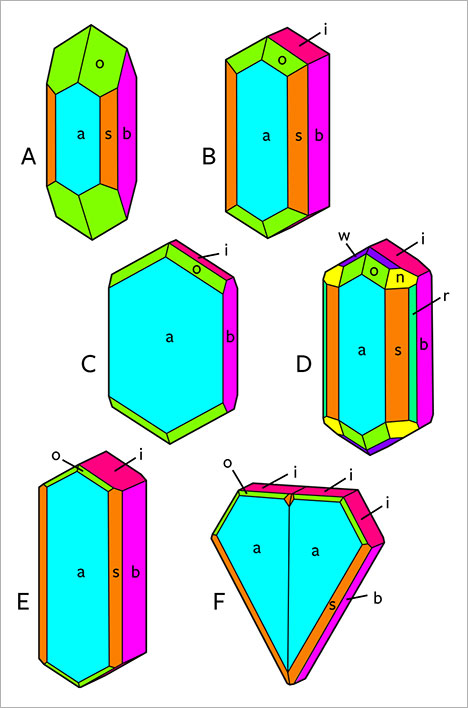
In a few samples of groups II and III, subordinate w and n dipyramids were also apparent (figures 12D, 13, and 14).
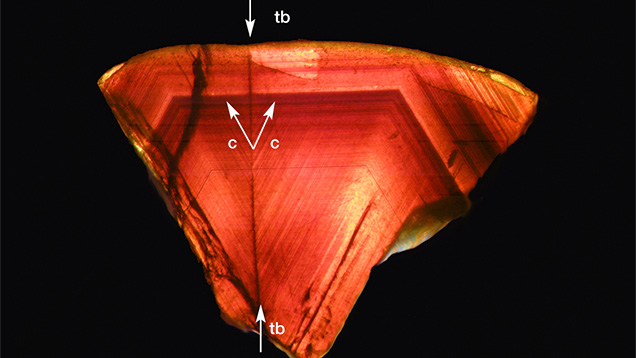
In certain crystals it was possible to see an additional series of planes inconsistent with the typically observed growth pattern. An example in which such a series of parallel lines crossed the normal growth pattern of a, o, w, and i planes is depicted in figure 15. In the particular example presented here, this series of additional planes was identified according to its orientation to other common growth planes and runs parallel to the dipyramid (114), a face observed as a growth plane neither in chrysoberyls from New England nor in crystals from other locations. The system of planes was not parallel to the common twin plane of chrysoberyl (031) either.

Chemical Composition. Along with the main components of chrysoberyl (beryllium, aluminum, and oxygen), all samples contained distinct amounts of iron as well as minor amounts or traces of boron, magnesium, titanium, gallium, niobium, and tantalum. The analyses are summarized in table 3, and a graphical representation is given in figure 16. For each of the group III samples, the analyses were subdivided into the following categories: whitish-appearing growth zones, designated “core” and representing prismatic i growth sectors; and yellow growth zones, designated “rim” and representing dipyramidal o and occasionally pinacoidal a growth sectors. An example of such a sample is depicted in figure 17. Augmenting the quantitative data obtained by LA-ICP-MS, X-ray fluorescence showed that all samples also contained traces of tin, but no suitable standard for quantitative determination of this trace element by laser ablation was available.
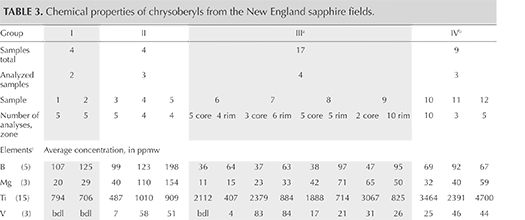
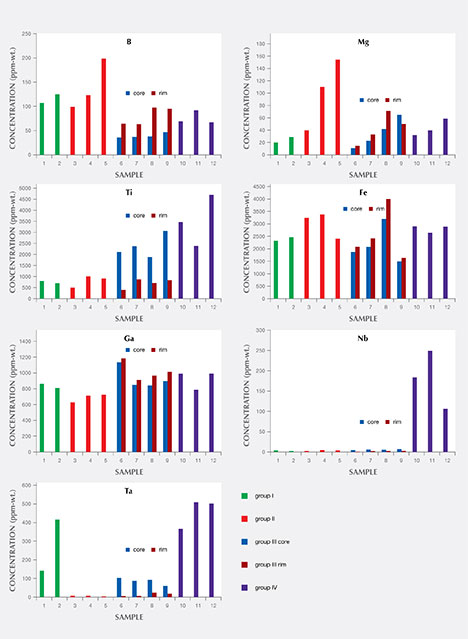
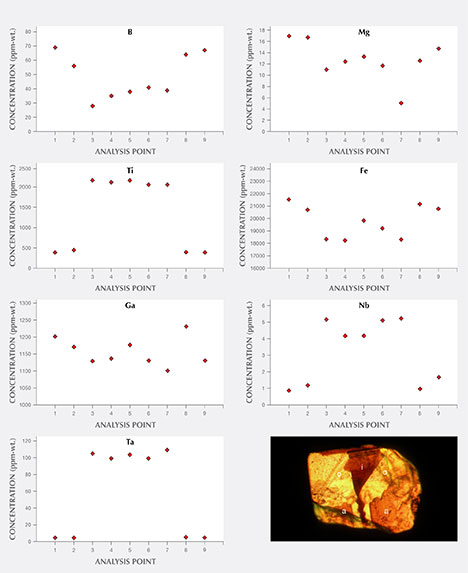
For the group III samples that showed a distinct sectorial zoning, the i growth sectors (core) always contained distinctly higher amounts of titanium, niobium, and tantalum, whereas moderately elevated amounts of boron, magnesium, and iron were found in the rim. Only a minor zoning was observed for gallium, with slightly elevated contents in the rim (figures 16 and 17). For the group I, II, and IV chrysoberyls, traverses of several analysis points measured across the samples revealed no significant zoning.
Comparing the different sample groups, boron levels in groups I and II were higher than in groups III and IV. Magnesium was highest in samples of group II, niobium was highest in group IV, and tantalum was highest in groups I and IV. Titanium was elevated in the whitish cores of group III and in samples of group IV, the latter likewise presenting a whitish appearance in reflected light. Iron and gallium showed no significant variation between the four groups.
Color, Pleochroism, and Spectroscopic Properties. In transmitted light, yellow samples from groups I and II and yellow growth zones of chrysoberyls belonging to groups III and IV showed no pleochroism. In contrast, the whitish growth zones seen in groups III and IV (yellowish brown or brown in transmitted light) exhibited a distinct pleochroism, with Y and Z showing light yellowish brown or brown and X displaying intense brown coloration.
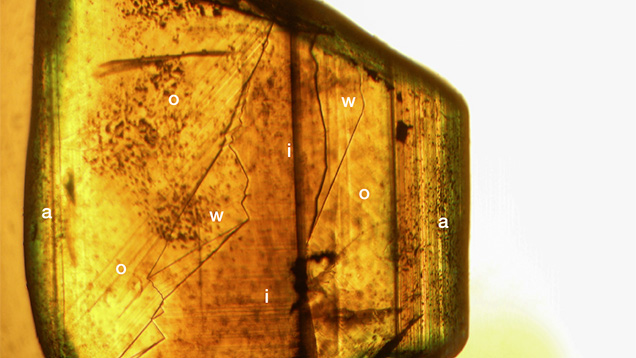
The whitish appearance in reflected light was observed in views parallel to the b- and c-axes (or in directions of view between the two axes) but not in a view parallel to the a-axis. Microscopic examination of cabochon-cut samples at high magnification revealed a dense pattern of needle-like inclusions oriented parallel to the a-axis (figure 18). These needles were responsible for the milky appearance and for the bright cat’s-eyes seen in cabochon-cut stones.

Absorption spectra were recorded for samples from all four groups. Selected samples are depicted in figure 19, and the corresponding spectra are displayed in the same figure.
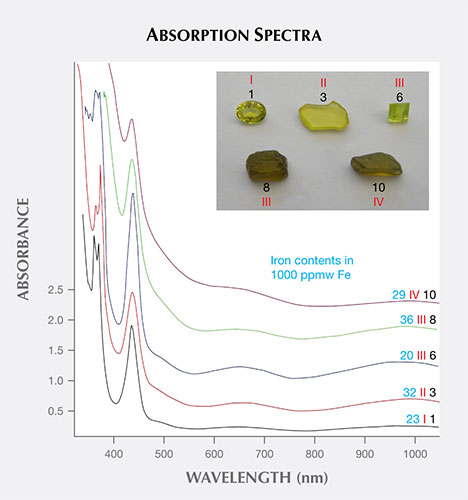
Spectra are presented for two yellow crystals, sample 1 (group I) and sample 3 (group II). The additional spectra provided were derived from zoned samples. Sample 6 (group III) had smaller whitish growth sectors, and sample 8 (group III) had larger whitish zones. Sample 10 (group IV) was primarily whitish with only small yellow growth sectors. As already mentioned, the growth zones appearing whitish in reflected light were yellowish brown or brown in transmitted light (again, see figures 8, 9, and 10).
The spectra of yellow samples from groups I and II (again, see figure 19) showed the commonly observed iron-related absorption spectrum for chrysoberyl, with weak absorption bands or shoulders at about 990, 650, and 500 nm, a strong band at 440 nm, and a strong doublet with maxima at 375 and 365 nm. Samples with yellowish brown to brown i growth sectors displayed these iron-related absorption bands, plus an additional absorption in the ultraviolet, extending into the visible range. Stated otherwise, a continuous absorption was present, starting in the red and increasing to the violet end of the visible region and into the ultraviolet range. This additional absorption was responsible for the brownish color component in transmitted light.
DISCUSSION
Chrysoberyl specimens recovered from the secondary sapphire deposits in Australia’s New England mining area were characterized by distinct mineralogical and chemical properties. Commonalities and variations among these properties enabled the samples to be summarized and presented according to four groups. These four groups might originate from different mines within the New England placer deposits, but it is also possible that the samples were separated from the sapphires mined commercially within one single area.
The morphology of the crystals, as determined mainly through internal growth structures, was comparable to the features of many chrysoberyls and alexandrites worldwide. Also common were the prismatic or tabular habits of the Australian stones.
Conversely, the most striking feature of the New England chrysoberyls was the strong sectorial zoning observed in a substantial number of the samples. Visual appearance in reflected and transmitted light, spectroscopic properties, and trace-element contents differed for prismatic i growth sectors as compared to the adjacent dipyramidal o growth zones. A pronounced sectorial color zoning between i (core) versus o and a (rim) growth sectors was recently described for alexandrites from Hematita, Brazil (Schmetzer and Hainschwang, 2012), but a pattern similar to the Australian material described here is unknown to the present authors. The series of structural planes intersecting the commonly observed growth pattern might represent glide planes that were caused by extremely high pressure during or after crystal growth.
Regarding chemical composition, the analytical technique used measured the bulk composition of the chrysoberyl host together with any minute inclusions present in specific areas with a diameter of approximately 50 µm (see figure 18). For the zoned crystals of group III, the greatest compositional contrast between the cores and the rims was the elevated titanium contents within the i growth sectors of the cores. Similarly high concentrations of titanium were also observed in samples of group IV, which were comprised primarily of i growth zones. These high titanium levels might correlate with minute needle-like inclusions. These tiny needles were responsible for the whitish appearance of the growth sectors in reflected light and caused chatoyancy in the cabochon-cut samples. The inclusions producing chatoyancy are normally described as rutile precipitates or extremely thin channels, but there exists no detailed study (e.g., by transmission electron microscopy) of this phenomenon in natural chatoyant chrysoberyl. In synthetic alexandrite, chatoyancy is produced by doping the melt with titanium oxide during crystal growth, followed by exsolution of needle-like precipitates through subsequent heat treatment of the as-grown crystals (Schmetzer et al., 2013b).
The yellow color apparent in many of the Australian samples studied here was related to minor amounts of iron, as confirmed by trace-element composition and spectroscopic data. The absorption spectra recorded were consistent with the literature (Farrell and Newnham, 1965; Pfenninger, 2000; Lottermoser et al., 2011). Iron is predominantly found as Fe3+ replacing Al3+ in both octahedrally coordinated sites of the chrysoberyl structure, but a small fraction of iron is also found occasionally in the bivalent state (Weber et al., 2007; Lottermoser et al., 2011).
The slightly greenish hue of some yellow samples might be due to the small traces of vanadium that were detected in various New England stones (see table 3). The influence of such small amounts of vanadium and/or chromium has been described recently for slightly greenish yellow chrysoberyls from Madagascar and Sri Lanka (Witthayarat and Thanasuthipitak, 2014).
The iron levels measured for both the cores and the rims of group III chrysoberyls as well as for the remaining samples of other groups were all in a comparable range (figures 16 and 17, table 3). Thus, different iron concentrations alone cannot be responsible for the color variation between yellow and brown samples or for the sectorial color zoning between cores and rims of group III stones. The distinct pleochroism within the brown sectors of groups III and IV suggests that an electron charge-transfer mechanism may cause the increasing absorption from red to violet in the visible range. A definitive answer, however, would require more extensive analysis, and an assignment of this particular absorption to Fe2+-Fe3+ or Fe2+-Ti4+ pairs is not possible at present.
Tin and gallium have long been recognized as trace elements in chrysoberyls and alexandrites from a variety of locations (Ottemann, 1965; Ottemann et al., 1978), and multiple trace elements in alexandrites have been used in recent studies to assist in origin determination (Malsy, 2010; Schmetzer and Malsy, 2011; Schmetzer et al., 2011). In these latter studies, a number of trace elements such as boron, magnesium, gallium, germanium, tin, and tantalum were considered, along with the main color-causing transition metals vanadium, chromium, and iron. The trace elements determined by LA-ICP-MS showed a wide compositional range for samples from Russia, Brazil, India, Sri Lanka, Tanzania, and Zimbabwe. Ternary diagrams prepared from the data then enabled separation between various localities.
Niobium and tantalum have been reported as trace elements in volcanic sapphires from placer deposits in Australia, including those of the New England gem fields, but also from basalt-related placer deposits in other countries such as Thailand, Laos, Cambodia, China, Nigeria, Madagascar, and Scotland. Nb or Ta contents are frequently at low levels, with ranges below 50 ppm, but concentrations of up to several hundred or several thousand ppm have been reported for sapphires from specific occurrences (e.g., the Weldborough area in Tasmania). The Nb and Ta contents measured in the corundum derive from various mineral inclusions, especially columbite, pyrochlore, ilmenorutile, and brookite. The sizes reported for such inclusions vary from the millimeter range down to the micron range, and some inclusions are even described as submicroscopic—i.e., near or below 1 µm in size (Coenraads, 1991; Guo et al., 1996; Sutherland et al., 1998, 2002, 2009; Saminpanya et al., 2003; Wathanakul et al., 2004; McGee, 2005; Abduriyim and Kitawaki, 2006; Zaw et al., 2006; Graham et al., 2008; Sutherland and Abduriyim, 2009; Upton et al., 2009; Pardieu, 2013).
Compared to the data published for volcanic sapphires in these prior works, however, some of the present chrysoberyl samples, while recovered from placer deposits related to Tertiary volcanics and unearthed together with basaltic sapphires, showed extremely elevated levels of niobium (group IV crystals) and tantalum (group I samples, cores in group III, and group IV chrysoberyls). Similarly high tantalum contents of up to 1364 ppm Ta were reported for Russian alexandrites but were not linked to any particular mineral inclusion (Malsy, 2010).
In considering the potential relationship of such elevated niobium and tantalum levels to the inclusion scene of the Australian stones here, many of the yellow samples from groups I and II as well as the yellow growth zones for groups III and IV did not show any inclusions in the immersion microscope. At high magnification, one chrysoberyl with a whitish area showed the small needle-like particles that were responsible for chatoyancy in cabochon-cut samples, but such inclusions have typically been described as rutile. Thus, further studies would be necessary to determine the mineral species present as inclusions and from which the elevated Nb and Ta could derive.
Recently, trace-element contents of corundum, chrysoberyl, and zircon grains recovered from the Mamfe placer deposit in southwest Cameroon were described (Kanouo et al., 2016). It was concluded that most of the blue, yellow, or grayish green corundum samples found in the Mamfe gem placer deposit were of magmatic origin and that the chrysoberyls were formed in granitic pegmatites; in other words, the sapphire and chrysoberyl grains originated from different host rocks. With respect to the chrysoberyls in particular, it was assumed that they had originated from two different granitic pegmatites; this conclusion was derived from analyses of four grains, on the basis primarily of variable trace-element ranges of tin, titanium, tantalum, niobium, and zirconium.
Regarding formation of the present four groups of chrysoberyl samples recovered from placer deposits in New South Wales, together with sapphires related to Tertiary volcanics (most likely volcaniclastic rocks), it is necessary to consider that granites and pegmatites are also located in the area (see, e.g., Audétat et al., 2000; Pettke et al., 2005; Schaltegger et al., 2005; Brown, 2006). However, chrysoberyls from these large granitic bodies are—to our knowledge—not mentioned in the literature, and therefore no direct comparison with such material is possible.
Thus, one of the following scenarios could explain each of the four groups:
- The chrysoberyls were formed in granitic pegmatites and subsequently accumulated at the placers without transport by or interaction with the volcanic rocks that carried the sapphires to the surface.
- The chrysoberyls and sapphires were accumulated from different host rocks in the earth’s crust and transported by volcanic activity together to the surface.
It is, of course, possible that the same underlying scenario does not apply to each of the groups of chrysoberyl samples described in this study. This could also be the reason for the widely ranging trace-element levels found in samples from the various groups. Conclusive data to decide between these possibilities, as worked out for corundum within the last two decades using trace-element contents, are not available for chrysoberyl.
CONCLUSIONS
The chrysoberyl crystals recovered together with sapphires related to Tertiary volcanics in the New England placer deposits were—according to highly variable chemical and mineralogical properties—subdivided into four groups. These groups might have originated, at least in part, from different host rocks. A possible transportation of the chrysoberyls together with sapphires to the surface by volcanic activity needs further study.
Samples from the largest of the four groups displayed a sectorial color zoning that is unique among chrysoberyl samples studied to date. The sectorial color zoning was seen in conjunction with variable trace-element contents between different growth sectors of the so-called core and rim, related mainly to prismatic i (011) or dipyramidal o (111) growth zones. Acicular inclusions producing chatoyancy in cabochon-cut samples were seen only in the cores. Compared to yellow samples or yellow growth sectors of zoned samples, only brown samples or brown growth sectors showed pleochroism. This latter feature, in addition to spectroscopic properties, indicated the presence of an additional cause for the brown color, beyond the trivalent iron responsible for the yellow color in the zoned chrysoberyls.



