Attractive Composite Quartz Beads

The measured RI was approximately 1.54. The beads displayed a patchy chalky blue reaction to short-wave UV and were inert to long-wave UV. Their Chelsea filter reaction was red, and under the desk-model spectroscope they displayed a band at approximately 650 nm. Under magnification, the blue areas displayed fluid inclusions that gave the beads a cloudy effect. In addition, the abundant fractures showed the presence of dye (figure 2) that was responsible for the beads’ blue color. Such features are commonly observed in dyed quartzite, but these were not sufficient to prove its identity. The golden brown areas, which consisted of fine flakes (again, see figure 2) held in a soft polymer, were later identified by EDXRF spectroscopy as copper and zinc composites. Similar composites in which the components are held together in a polymer matrix have been known in the trade for several years now (see G. Choudhary, “A new type of composite turquoise,” Summer 2010 G&G, pp. 106–113). Also present were thick layers of polymer along the edges of the blue and golden brown areas, which appeared green due to the overlap of the blue dye with the golden flakes. Raman spectroscopy confirmed that these blue portions were quartz.
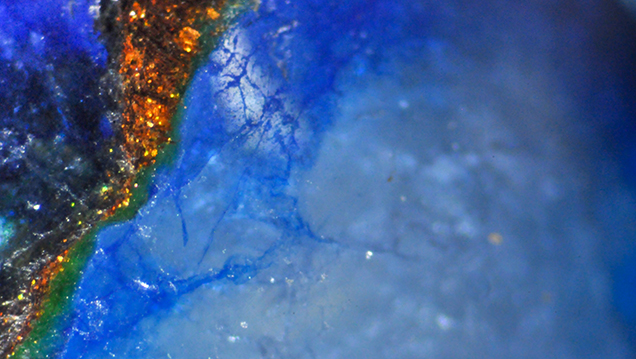
Figure 2. Color concentrations of blue dye were easily visible along the fractures against a white background, while the golden brown areas were composed of copper-zinc based fine flakes. Also note the greenish polymer along the edge of the golden brown and blue areas. Photomicrograph by Gagan Choudhary; magnified 48×.
Recognizing these beads as composites was straightforward, but identification of the components required Raman and EDXRF spectroscopy. Although these beads were obviously created to offer something fancy and attractive to consumers at a low price, clear and complete disclosure remains imperative. 


