Gem Characterization

3161 cm–1 Infrared Feature in Synthetic Sapphires
Gagan Choudhary and Sandeep Vijay
Gem Testing Laboratory, Jaipur, India
The 3161 cm–1 mid-infrared (IR) spectral feature (figure 1) is an important tool in the identification of unheated sapphires, especially in material from low-iron metamorphic environments such as Sri Lanka. This feature is a series of bands, composed of a strong peak at ~3161 cm–1 and smaller side bands at ~3075, 3240, and 3355 cm–1. A few researchers have attributed these features to OH groups involved in charge compensation with Si4+, while some have assigned them to structurally bonded OH, associated with Mg2+. The 3161 cm–1 series is more commonly observed in natural-color yellow sapphires than any other color of corundum; occasionally it is encountered in blues and pinks. These authors, however, have encountered a strong feature at ~3161 cm–1 in a few specimens of yellow synthetic sapphire grown by the flame-fusion (Verneuil) process. Their synthetic origin was determined on the basis of inclusion study, which revealed the presence of clouds of minute gas bubbles, along with some bomb-shaped gas bubbles, typically associated with corundum or spinel grown by the flame-fusion process. Milky zones of fine pinpoints and a “plato” effect were also present.
The 3161 cm–1 mid-IR feature in these synthetic yellow sapphires displayed side bands at approximately 3220 and 3277 cm–1, as opposed to 3240 and 3355 cm–1 in natural sapphires.
Agate Analysis by Raman, XRF, and Hyperspectral Imaging Spectroscopy for Provenance
Aaron Celestian1, Arlen Heginbotham2, Rebecca Greenberger3, Bethany Ehlmann3, Bibek Samanta4, Alyssa Morgan1, and Sergey Mamedov5
1Natural History Museum of Los Angeles County
2J. Paul Getty Museum, Los Angeles
3California Institute of Technology, Pasadena
4University of Southern California, Los Angeles
5Horiba Scientific, Edison, New Jersey
The Getty Institute in Los Angeles recently acquired the Borghese-Windsor Cabinet (figure 1, left), a piece of furniture extensively decorated with agate, lapis lazuli, and other stones. The cabinet is thought to have been built around 1620 for Camillo Borghese (later Pope Paul V). It was traditionally thought that all agate gemstones acquired during the sixteenth and seventeenth centuries were sourced from the Nahe River Valley near Idar-Oberstein, Germany. While Brazilian agate began to be imported into Germany by the 1800s, it is possible that some was imported in the eighteenth century or earlier. A primary research goal was to determine if the agates in the Borghese-Windsor Cabinet are of a single origin, or if they have more than one geologic provenance.

Both quartz and moganite will crystallize together as agate forms, but moganite is not stable at Earth’s surface and will convert to quartz over tens of millions of years (Heaney, 1995; Gíslason et al., 1997; Moxon and Rios, 2004). Thus, older agate contains less moganite. Agate from Idar-Oberstein is Permian in age (around 280 million years old), while agate from the Brazilian state of Rio Grande do Sul generally formed during the Cretaceous (around 120 million years ago). It is thought that Rio Grande do Sul would have been a primary source of material exported to Europe because it is one of Brazil’s oldest and largest agate producers.
When examining the cryptocrystalline parts of agate from comparative collections, Brazilian agates from the collection of the Natural History Museum of Los Angeles County (NHMLA; figure 1, right) had 8% or higher moganite concentration, whereas the Idar-Oberstein agate (on loan from the Smithsonian National Museum of Natural History) had less than 2% moganite. The moganite distribution in the agate is heterogeneous, likely due to different growth stages and changing geological conditions during agate formation. Using the Raman maps, we were able to isolate the areas that contained moganite + quartz and measure the ratios in those specific bands (figure 2). This narrow-band approach to determining quartz to moganite ratio, when compared to broad-brand and whole-sample approaches, was shown to be more reproducible in distinguishing Brazilian from German agates.

These same agates from the Brazilian and German localities were then taken to Caltech to collect hyperspectral imaging data (on a custom-built Headwall Photonics co-boresighted visible/near-infrared and shortwave infrared sensor). Imaging data were compared to the NHMLA laboratory Raman and X-ray fluorescence analyses, and correlation analysis of combined datasets from the three different experimental procedures allowed us to establish a unique characterization pattern for the different localities.
REFERENCES
Gíslason S.R., Heaney P.J., Oelkers E.H., Schott J. (1997) Kinetic and thermodynamic properties of moganite a novel silica polymorph. Geochimica et Cosmochimica Acta, Vol. 61, No. 6, pp. 1193–1204, http://doi.org/10.1016/S0016-7037(96)00409-7
Heaney P.J. (1995) Moganite as an indicator for vanished evaporites: A testament reborn? Journal of Sedimentary Research, Vol. 65, No. 4A, pp. 633–638, http://dx.doi.org/10.1306/D4268180-2B26-11D7-8648000102C1865D
Moxon T., Ríos S. (2004) Moganite and water content as a function of age in agate: An XRD and thermogravimetric study. European Journal of Mineralogy, Vol. 16, No. 2, pp. 269–278, http://dx.doi.org/10.1127/0935-1221/2004/0016-0269
Beryllium Heat Treatment of Blue Sapphire from Sri Lanka
Sutas Singbamroong1,2, Panjawan Thanasutthipitak1, Thawatchai Somjaineuk3, and Nazar Ahmed2
1Department of Geological Science, Chiang Mai University, Thailand
2Dubai Central Laboratory Department, Dubai, United Arab Emirates
3Chanthaburi Gem and Jewelry Manufacturer Association, Chanthaburi, Thailand
Since at least 2000, corundum has been subjected to a beryllium (Be) heat treatment technique in Chanthaburi, Thailand. For this study, samples of transparent to translucent milky-white to yellow, purple to violet, and light to medium blue sapphires from Sri Lanka (metamorphic origin) were heat treated with Be in three types of furnaces (gas, electric, and fuel) at various temperatures and in both oxidizing and reducing atmospheres. The technique of Thai gem heating specialist Thawatchai Somjaineuk was used to intensify blue color, improve clarity, and distribute uneven color. Somjaineuk’s technique has been used to enhance Sri Lankan corundum with a milky/silky appearance since 2004, and supplies approximately 50 kg of beryllium-treated blue sapphire per year to the gem market.
The samples were studied after each step of heating for basic gemological properties, spectroscopic properties using ultraviolet/visible/near-infrared (UV-Vis-NIR) and Fourier-transform infrared (FTIR) absorption spectroscopy, and chemical composition using laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS). The corundum samples were first heated in a traditional O2/LPG mixed-gas furnace to about 1500°C for two hours in an oxidizing atmosphere. The white to yellow and light to medium blue sapphires turned colorless, whereas the purple to violet sapphires became pink. The second step of heating was performed with Be in an electric furnace at about 1700°C for 48 hours in an oxidizing atmosphere. After this process, the milky/silky colorless sapphires became a more transparent yellow, while the pink sapphires turned orange-pink. These stones were enhanced in the final step by reheating in a fuel furnace at about 1700°C for 72 hours in a reducing atmosphere. All samples became blue with light to strong saturation and tone.

The combination of the color appearance, the absorption spectra analyzed after oxidation with Be and reduction heating (figure 1), and the chemical data suggest that Be and/or Mg trapped-hole yellow color centers—created during oxidation heating with Be—were made inactive after reduction heating. The blue coloration is mainly caused by strong broad absorption bands of Fe2+/Ti4+ intervalence charge transfer (IVCT) mechanism without Fe2+/Fe3+ IVCT. However, chemical data were analyzed for those samples and showed relatively high Mg and Be concentrations in comparison with the Ti composition, which does not fit well with the model that indicates [Ti4+] > [Mg2+ + Be2+] causes blue coloration. This heating technique is still not well understood. Further experiments and analyses are being carried out to confirm the role of beryllium in blue sapphires.
Gemological and Spectroscopic Characteristics of Australian Sapphires
Yafen Xu and Jingru Di
Gemmological Institute, China University of Geosciences, Wuhan
Although Australia has assumed a major role in the production of sapphire, research on this material has not been comprehensive. This study aims to analyze the gemology and spectroscopy of Australian sapphires and provide a theoretical basis for their treatment.
Under the optical microscope and other conventional instruments, hexagonal color zones were blue and yellow. Healing fissures and inclusions were extremely common. Raman spectroscopy showed that the inclusions were two-phase: CO2 and H2O with sapphire, rutile, zircon, diaspore, and amphibole, among others. Sulfur on the healing fissures indicated that S was filled during transportation. The IR spectra of the Australian sapphires typically revealed a 3310 cm–1 absorption peak (figure 1, left). This absorption feature is related to structural OH groups within the sapphire and revealed that these samples grew in reducing conditions. LA-ICP-MS indicated that the Cr/Ga ratio was less than 1 and the Fe/Ti ratio was generally 10–100 (figure 1, right), the typical ratio of magmatic sapphire. The iron content was between 3230 and 9431 ppm. Color varied with the content of Fe, Ti, Si, and Mg. UV-Vis absorption peaks (figure 2) at 377, 387, and 450 nm were caused by the d-d electronic transition of Fe3+ and Fe2+-Fe3+ in the region with less Ti; the absorption band centered at 559 nm in the yellow-green region indicated the charge transfer of Fe2+-Ti4+→Fe3+-Ti3+ and higher Ti content in this area. Fe2+-Fe3+ charge transfer often occurs together with Fe2+-Ti4+→Fe3+-Ti3+ charge transfer and causes the wide absorption band at 700–800 nm centered at 754 nm. The center may shift with different ratios of the charge transfer of Fe2+-Fe3+ and Fe2+-Ti4+→Fe3+-Ti3+.


REFERENCES
Kan-Nyunt H.-P., Karampelas S., Link K., Thu K., Kiefert L., Hardy P. (2013) Blue sapphires from the Baw Mar mine in Mogok. G&G, Vol. 49, No. 4, pp. 223–232, http://dx.doi.org/10.5741/GEMS.49.4.223
Yimiao L., Tao C. (2015) Gemmological characteristic of ruby and sapphire from Muling, Heilongjiang Province. Journal of Gems & Gemology, Vol. 17, No. 4, pp. 1–7.
A Grading Method of Jadeite Jade Transparency Based on Digital Image Analysis
Danlu Cui
Gemmological Institute, China University of Geosciences, Wuhan
In the jadeite jade market, transparency is an important feature judged by experienced practitioners observing with the unaided eye under reflected light. However, this method is easily influenced by subjective factors.
This research simulates human observation of characteristics that could help in judging jadeite transparency through certain visual information features. This approach could allow gemologists to evaluate transparency rapidly while effectively avoiding subjective factors, especially under the same test conditions used to judge jadeite jade color.
Some promising research results have been obtained, through comparing the lightness value of each image pixel in jadeite jade pictures and then using the maximum between-class variance method (OSTU) to obtain a binarization threshold. Thus, the image data is classified into a relatively bright area and a relatively dark area. When a beam of light is directed across an oval-cut jadeite jade with different degrees of transparency, different results are obtained from the images.
- In oval-cut jadeite jade with a high degree of transparency, a beam of light arrives at the underside and then converges in the other side of its curved surface, forming a relatively bright area.
- In oval-cut jadeite jade with a medium degree of transparency, a beam of light is divided into two parts—one part reflected at the point of incidence or absorbed during the light transmission, and the other part arriving at the underside and then converging in the other side of its curved surface. Therefore, the brightness of the whole image is even.
- In oval-cut jadeite jade with a low degree of transparency, most of the light is reflected at the point of incidence or absorbed. Little light reaches the underside, and therefore a relatively dark area forms at the other side of the curved surface.
Based on these three characters, oval-cut jadeite jade with different degrees of transparency can be judged objectively and automatically.
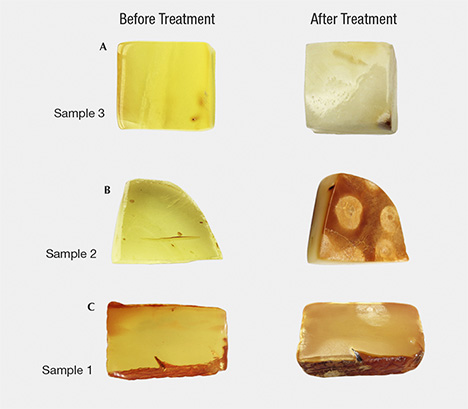
Inclusion Characteristics of Wax-Like Amber After Hydrothermal Treatment
Yamei Wang1,2 and Yan Li1 (presenter)
1Gemmological Institute, China University of Geosciences, Wuhan
2Gem Testing Center of China University of Geosciences, Wuhan
Amber products can be hydrothermally treated (figure 1) in order to improve the transparency of the material. In this process, an abundance of tiny nano- or micro-sized bubbles penetrate the amber in the presence of an aqueous solution (with some catalyst) through controlling the temperature and pressure and selecting an inert atmosphere environment (figure 2). After the treatment, the inner layer of the weathered skin of rough amber material will generate a layer with various thickness of a yellowish white or greenish yellow “hydrothermally treated skin” or a corrugated crust containing pores. The finished amber shows residues with white hydrothermally treated spots of various sizes, which may enter into the amber’s interior or remain on the finished surface. The treated wax-like amber displays abundant gas-liquid inclusions with small and dense flat or disc-shaped bubbles accompanied by tiny stress fracture patterns. The bubbles are uneven in size and densely distributed, forming a cloud-like effect. Because the infrared spectra data of the experimental samples before and after the treatment showed little difference, identification required the support of statistical FTIR data. A series of comprehensive tests are needed to identify hydrothermally treated amber, including the diagnostic evidence of the crust-like skin containing spots.

Interesting Inclusions in Peridot from Jilin, China
Zhiqing Zhang, Min Ye, and Andy H. Shen
Center for Innovative Gem Testing Technology
Gemmological Institute, China University of Geosciences, Wuhan
Recently we received 50 gem-quality rough peridot from the Yiqisong Nanshan olivine ore district, a new mineral occurrence in Dunhua City in China’s Jilin Province. These peridot crystals exhibit a yellowish green hue, rather high transparency, and some visible inclusions. Study of the inclusions showed interesting results (figure 1 left, A–D).

For precise observation and accurate testing, the crystals were windowed and doubly polished. Comprehensive microscopic and Raman spectroscopic analysis indicated the following typical inclusions: “lily pads,” round transparent inclusions, strongly colored minerals with or without healed secondary fractures, green crystals of enstatite and diopside, and a rare dark mineral inclusion of lizardite, identified by Raman spectroscopy and by referencing the RRUFF database (figure 1, right). Further gemological research is being carried out on these samples, and more data will be published in a full article.
IR Absorption Spectrum of Type Ib HPHT-Created Diamonds as an Indicator of Their Growth Conditions
Viktor Vins1, Alexander Yelisseyev2, Dmitry Bagryantsev1, and Alex Grizenko3
1Velman, Ltd., Novosibirsk, Russia
2Sobolev Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia
3Lucent Diamonds, Inc., Los Angeles
Donor nitrogen atoms are the simplest crystal lattice defect, and their one-phonon absorption spectrum is well studied. The spectrum shows two main features: a main band at 1130 cm–1 and a narrow peak at 1344 cm–1. The absorption intensity and exact position of these two peaks give information about diamond growth conditions and crystal lattice perfection. In particular, our results showed that a decrease in growth rate corresponded to a decrease in the μ1130/μ1344 absorption intensity ratio. The ratio decreased from 2 to 1.5 in samples grown in the Fe-Ni-C system and from 1.64 to 0.95 in samples grown in the Fe-Co-C system. Since the μ1130/μ1344 ratio is sensitive to growth conditions, it could serve as a criterion for diamond quality, showing the content of impurity defects as well as the amount of internal tensile stress.
Studying isotopically modified diamonds is also informative. For example, if the carbon part of the growth system was 50% 12C graphite and 50% 13C graphite, an isotopic shift at 1344 cm–1 was observed, while the main band at 1130 cm–1 did not shift. The samples containing 15N isotope, conversely, revealed a 15 cm–1 shift toward long wavelengths of the 1130 cm–1 band, whereas the 1344 cm–1 peak remained at its frequency. It can be concluded that the 1130 cm–1 band is associated with the resonant vibrations of the N-C bond, while the 1344 cm–1 peak is related not to the donor nitrogen atom but to local vibrations of the carbon atom, which is bonded to the unpaired electron of the impurity nitrogen. The position of the Raman peak on the spectra taken at different points of the sample with 47% 13C showed that the biggest shift of the diamond peak (ν = 1312.8 cm–1) was seen in parts of the crystal immediately adjacent to the seed region. The Raman peak varied from 1321.1 to 1322.5 cm–1 in other parts of the sample, which corresponds to 25 ÷ 27% of 13C. The full width half maximum (FWHM) of the Raman peak was the largest (7.8 cm–1) at ν = 1312.8 cm–1. In all other points it ranged from 6.2 to 7.4 cm–1. In addition to an unusually high μ1130/μ1344 intensity ratio, which in “traditional” nickel-containing diamond ranges from 1.5 to 2.0, this indicates that isotopically modified diamonds have a rather imperfect crystal lattice. This could be caused by internal stress resulting from the incorporation of an isotope with a larger atomic size than that of 12C.
This work was supported by grant 16-05-00873a from the Russian Foundation for Basic Research and by the state assignment project 0330-2016-0006.
Laue X-Ray Backscatter Spot Patterns: A Novel Way of Identifying Various Gemological Crystals
Hollis Milroy and Sandra Hektor
University of Toronto
Although Laue X-ray backscatter imaging of crystals dates back to the 1920s, the application of this technique to the study of gemology is very much a new concept. This study investigates the use of Laue backscatter spot patterns (also called Laue-grams) to positively identify several gem crystals of varying crystal structure and atomic complexity. Approximately 50 exposures were taken using a tungsten filament running at 2000 W (50 kV × 20 mA), with the resulting Laue backscatter images captured on medical X-ray film. Using a high-resolution scanner, the spot patterns from the developed film were compared to computer-generated models, which agreed with the film images to a high degree of accuracy.
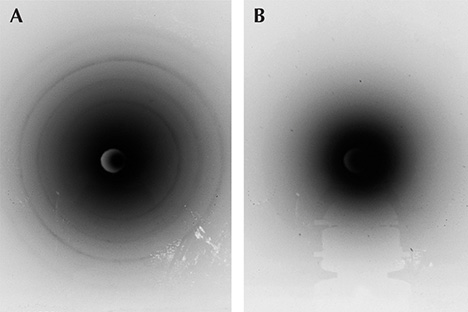
Exposure times and crystal orientations relative to the X-ray beam axis were varied. Control exposures were run to ensure background effects (e.g., from the sample holder) did not contribute at all to the spot patterns.
The advantage of the noninvasive and nondestructive Laue backscatter method is that material can be identified from any orientation of the crystal. This is helpful when gemstones are embedded in jewelry or other materials such as rocks. Furthermore, anomalies from crystal impurities will appear on the film proportionately: The spot pattern of the predominant material will appear most visible and thus always easily determinable. For instance, the small impurities that produce different-hued gemstones will not affect the produced spot pattern from the underlying crystal structure.
Multifunction Spectrometer for Color Grading and Identification of Diamonds and Gemstones
Yan Liu
Liu Research Laboratories, South El Monte, California
A multifunction dual integrating sphere spectrometer has been developed for color grading and identification of diamonds and gemstones. The spectrometer has nine functions: spectral reflectance measurement, spectral transmittance measurement, color measurement, UV fluorescence measurement, photoluminescence (PL) measurement, color grading of gemstones, color grading of colored diamonds, color grading of jadeite, and grading of alexandrite effect. The color grading by artificial intelligence and the fine spectral measurement of PL at room temperature are particularly useful for gemological laboratories and the jewelry industry. The artificial intelligence for color grading includes a neural network and a fuzzy logic algorithm. The PL measurement is calibrated by a NIST-traceable lamp to measure the relative spectral distribution of photoluminescence, and a mathematic algorithm is developed to obtain a fine PL spectrum at room temperature. In addition, the photoluminescence measurement can amplify a weak spectrum up to 1,000 times for gemological research and identification purposes. The function for color grading of jadeite has adjustments for color area percentage and shape curve for accurate color grading of carved pieces. The grading of alexandrite effect is calculated under five standard CIE illuminants: A, D65, F3, F7, and F11. The spectrometer can be used to perform most tasks of color grading, spectroscopic identification, and research in advanced gemological laboratories.
Nephrite from Luodian, Guizhou Province of China
Quanli Chen1, Xianyu Liu1, 2, Haitao Wang3, Wenjing Zhu1, Sujie Ai1, and Zuowei Yin1
1Gemmological Institute, China University of Geosciences, Wuhan
2College of Jewelry, Shanghai Jian Qiao University, Shanghai
3School of Jewelry, Guangzhou College of South China University of Technology, Guangzhou
In recent years, a new variety of nephrite has been discovered in Luodian County in Guizhou Province, China (figure 1). It lacks greasy luster but possesses a distinctive “porcelain” luster and a fine texture. The white samples tend to show a more or less gray hue.

Samples with a white to bluish white colors were studied via X-ray fluorescence (XRF), X-ray diffraction (XRD), ICP-MS, and FTIR. The results reveal that Luodian nephrite (figure 2), whose SG ranges from 2.77 to 2.90 and is slightly lower than that of Hetian nephrite, is mainly composed of tremolite, and parts of the samples contain a small amount of quartz. The chemical constituents of Luodian nephrite are SiO2 (56.75–59.01 wt.%), MgO (23.69–25.03 wt.%), and CaO (11.25–12.00 wt.%, lower than the standard value of tremolite).

Among the trace elements of Luodian nephrite, Ti, V, Cr, Mn, and Co show higher relative mass fraction, with Ti ranking top (relative mass fraction: 2.05–4.52 × 10–8). The content of these elements is exceptionally low in Hetian white nephrite, which may shed some light on the gray hue of Luodian nephrite. Light rare earth elements are relatively concentrated, and Eu shows both positive and (more frequently) negative anomalies. Compared with Hetian white nephrite, the total content of rare earth elements of Luodian nephrite is higher, especially the elements La, Nd, Sm, Tb, and Er, indicating that the ore-forming environment is different between the two localities.
Observing the microstructure characteristics using scanning electron microscopy (SEM) revealed that the tremolite in Luodian nephrite has a mainly fibrous crystalline structure, and the various microstructure features of the aggregate are based on the different aggregation modes of fibrous tremolite. Therefore, the fibrotic range, geometric size, form combination, interaction of the tremolite, and tightness of polymerization are the basis of quality in Luodian nephrite.
“Raindrop” Turquoise from China
Ling Liu, Mingxing Yang, Yan Li, Yalun Ku, Jia Liu, and Zenmin Luo
Gemmological Institute, China University of Geosciences, Wuhan
Recently, a new type of turquoise with randomly distributed spots (figure 1A) from Hubei Province, China—where there are abundant gem-quality turquoise mines—has become popular with jewelry consumers and collectors. The material is called “raindrop turquoise” because of the unique inclusions with blue, blue-green or (rarely) yellow color. The raindrop-like inclusions and the substrate always display a similar hue, but the color of the raindrop-like inclusions is much deeper, and their transparency and hardness are higher.
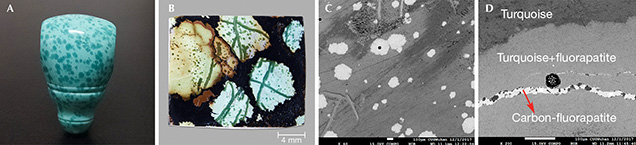
It appears that so-called raindrop turquoise has not been well studied. Several typical specimens from the jewelry market were systematically investigated for their mineral compositions and spectral characteristics using micro-XRD, micro-FTIR, Raman, and electron microprobe analysis. The raindrop inclusions (figure 1B) were mainly composed of turquoise and fluorapatite, while the substrate was mainly turquoise. The Raman spectra of the raindrop inclusions showed the characteristic peaks of turquoise, with strong and sharp peaks at 965–968 cm–1 caused by PO43– of fluorapatite. In addition, a weak shoulder at 1070 cm–1 was observed in some samples because of CO32– replacing PO43– (Awonusi et al., 2007). The micro-FTIR spectra of the raindrop inclusions showed double weak peaks near 1460 cm–1 and 1430 cm–1, resulting from the asymmetric stretching vibration of CO32– (Fleet, 2009). The backscattered electron images (figure 1C) showed that the gray between the raindrop and the substrate was distinct, indicating the difference in their mutual mineral phases. Some raindrop inclusions kept the perfect hexagonal shapes of apatite (again, see figure 1C). In addition, a lighter and narrower line (~10 μm) was also observed in the vein of the sample (figure 1D). The chemical compositions of the raindrops and veins were Al2O3, P2O5, CaO, CuO, FeOT, and F, while the narrow line mainly consisted of P2O5, CaO, and F.
From the X-ray diffraction (XRD) and Raman results, the raindrop and vein inclusions are mixed with turquoise and fluorapatite, while the lighter and narrower line is pure carbon-fluorapatite. Hence, the deeper color of raindrop-like inclusions can be attributed to the mixture of turquoise and fluorapatite/carbon-fluorapatite.
REFERENCES
Awonusi A., Morris M.D, Tecklenburg M.M. (2007) Carbonate assignment and calibration in the Raman spectrum of apatite. Calcified Tissue International, Vol. 81, No. 1, pp. 46–52, http://dx.doi.org/10.1007/s00223-007-9034-0
Fleet M.E. (2009) Infrared spectra of carbonate apatites: v2-region bands. Biomaterials, Vol. 30, No. 8, pp. 1473–1481, http://dx.doi.org/10.1016/j.biomaterials.2008.12.007
Tianhuang Stone—The Most Valuable Seal Stone in China
Tao Chen1, Yungui Liu2, and Quanli Chen1
1Gemmological Institute, China University of Geosciences, Wuhan
2State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Wuhan
Tianhuang stone, from the village of Shoushan in Fujian Province, is the most precious Chinese seal stone. It has a profound cultural heritage in China and is quite expensive. Therefore, systematic research on the identifying gemological and mineralogical characteristics of Tianhuang stone has important value implications. Tianhuang stone mainly has a yellow color, though it can sometimes be white, red, or black. Most Tianhuang stones have weathered skins, red cracks, and “turnip” inclusion veins.

According to X-ray diffraction and FTIR results, Tianhuang stone can be divided into three types: dickitic, nacritic, and illitic. Dickitic-type Tianhuang stone can be further divided into ordered dickitic and disordered dickitic. Illitic-type Tianhuang stone is usually composed of 2M1 illite and a small amount of 1M illite.
The weathered skin and matrix have the same mineral composition. LA-ICP-MS results suggest that iron content is higher in the skin than in the matrix. These findings, along with total iron analyses, indicate that the yellow color of Tianhuang stone is caused by Fe3+ ions in the crystal structure of dickite, nacrite, or illite. Red cracks are caused by iron minerals that entered along the cracks. The iron minerals display three forms: thick film-state, short needles, and film or fine granular, as observed by scanning electron microscopy (SEM). The “turnip” vein is a kind of fine, white spiderweb inclusion in Tianhuang stone, which has no obvious boundary between matrix and vein. SEM, energy-dispersive spectrometry (EDS), and laser Raman microspectrometry results show that turnip vein is composed of svanbergite.
Unusual “Vorobyevite” Beryl from Afghanistan
Yang Hu and Ren Lu
Gemmological Institute, China University of Geosciences, Wuhan
Beryl is an important gem species that includes goshenite, aquamarine, emerald, heliodor, morganite, and red beryl varieties. An unusual blue beryl sold as “vorobyevite” or “rosterite” is sometimes seen on the market. This crystal has a special hexagonal tabular morphology, sometimes concave, convex, or fibrous-like on basal pinacoids. The material is mined from the Deo Darrah, Khash, and Kuran Wa Munjan districts in Badakhshan Province, Afghanistan. It most likely formed in granitic pegmatite, as evidenced by the associated dark blue tourmaline and spherical muscovite aggregates (figure 1, left). Characteristics of an Afghan vorobyevite sample and the use of the term are discussed here.

This sample was pale blue with a yellow hue in the center. Parallel intergrowth and a hexagonal growth texture were shown on a two-sided basal face. Dichroism was medium, appearing pale blue along the ordinary (o-) ray and blue along the extraordinary (e-) ray. The fluorescence response was inert to both long-wave and short-wave UV. The sample had a refractive index (RI) of 1.570–1.575 and a specific gravity (SG) of 2.53. Abundant two-phase inclusions with various shapes were observed (figure 1, right). All of these gemological properties were in agreement with aquamarine.
LA-ICP-MS analysis showed that alkali content in the form of Na and Cs (Cs2O 0.03–0.04 wt.%, and Na2O 0.07–0.08 wt.%) was relatively low according to our chemical database of all beryl varieties, classified as “low-alkali” beryl in agreement with the dominance of type I water in structural channels revealed by Raman and IR spectra. In addition, Ge Sn, Nb, and Ta were relatively rich compared with aquamarine from other origins. Due to few substitutions in the Al octahedral and Be tetrahedral crystal site, this Afghan sample was identified as “normal beryl.” UV-Vis-NIR spectroscopy showed absorptions of Fe ions (FeO 0.56–0.60 wt.%) at 372, 425, 600, and 820 nm, and the blue color was stable under sunlight. Therefore, the blue color was attributed to Fe ions rather than natural or artificial irradiation. A yellowish area in the center of the sample indicated that the blue color was natural.
The term “vorobyevite” was first applied to colorless to yellowish rose beryl containing large amounts of lithium and cesium (Cs2O 3.04 wt.% and Li2O 1.43 wt.%) from Lipovka, Russia (Yakubovich et al., 2009). The term “rosterite” designated a cesium beryl of colorless or pink color from Elba, Italy, almost synonymous with “vorobyevite” (Hänni and Krzemnicki, 2003). But neither term is officially accepted by the International Mineralogical Association or normally used for a beryl variety. Based on gemological, spectroscopic, and chemical characteristics, we confirmed this Afghani vorobyevite belonged to the beryl species, specifically the low-alkali aquamarine variety. However, similar tabular beryls from Mogok (Myanmar) and Sichuan (China) with a pale blue to dark blue color (see figure 1, left) were identified as “alkali-rich” aquamarine in our study. Although the tabular morphology in this Afghan sample was quite uncommon, tabular morphology is not a criterion for identifying vorobyevite or rosterite.
REFERENCES
Hänni H.A., Krzemnicki M.S. (2003) Caesium-rich morganite from Afghanistan and Madagascar. Journal of Gemmology, Vol. 28, No. 7, pp. 417–429.
Yakubovich O.V, Pekov I.V, Steele I.M, Massa W., Chukanov N.V. (2009) Alkali metals in beryl and their role in the formation of derivative structural motifs: Comparative crystal chemistry of vorobyevite and pezzottaite. Crystallography Reports, Vol. 54, No. 3, pp. 399–412, http://dx.doi.org/10.1134/S1063774509030067
Young Blue Sapphires from Muling, China
Yimiao Liu and Ren Lu
Gemmological Institute, China University of Geosciences, Wuhan
The area around Muling, a small town located in northeastern China, produces attractive corundum in a wide variety of color, clarity, and sizes (Liu and Lu, 2016), along with gem-quality pyrope, zircon, and black spinel. Some of its facet-quality sapphires (figure 1) are comparable to those from the finest sapphire localities in the world.

Sapphire from Muling is mostly extracted from alluvial (or secondary) deposits. Information on genesis, general formation environment, and deposit type is still incomplete and being researched to support efficient exploration.
Muling is distributed along the northern section of the Dunhua-Mishan Fault, which is a lithospheric extension between the Xing-Meng orogenic belt and the Pacific subduction zone (Ling et al., 2017). The volcanic eruptions of the Dunhua-Mishan graben began at about 44.9 Ma and ended about 5,140 years before the present (Wang et al., 2001). Gemstones were found in the placer derived from Miocene (5.3–23 Ma) basalt, which is regarded as the gem carrier. The corundum-bearing layers underlie a few meters of soft sandy clay and fertile topsoil.
This study arose from a preliminary examination of 14 faceted and 74 rough corundum from the Muling deposits. During examination of the internal characteristics, we found zircon inclusions in three blue sapphires. As one of the best geochronometers, zircon provided an initial estimate of the formation age through U-Pb dating methods.
Zircon inclusions were identified by laser Raman spectroscopy. Euhedral crystals with no evidence of surface corrosion or oxidation could indicate that the zircon inclusions within the host sapphire were syngenetic and nearly unaffected by radiation (called “high” zircon in gemology). Therefore, the ages obtained on zircon inclusions are in equilibrium with the initial formation time of the host sapphire.
U-Pb dating analyses of zircon inclusions were conducted using LA-ICP-MS at the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Wuhan. Due to the small size of the target inclusions (approximately 150 μm × 50 μm) and unavoidable interference from common lead, only some subsets of information could be preserved for the age calculation. The analyses of zircon inclusions gave 206Pb/238U ages ranging from 8 to 10 Ma (i.e., Miocene). Being completely enclosed within the host sapphires, the transparent euhedral zircons were protected throughout their history from interaction with permeating fluids. Thus, the obtained ages correspond to the formational ages of these sapphires. Combined with the geologic settings, this time period would have allowed numerous volcanic events to deliver the corundum as xenocrystal from the source to the top. The formation condition of Muling sapphires might be associated with the Miocene volcanic events.
The U-Pb ages of zircon inclusions (along with a full range of trace elements within zircon) can also provide distinctive provenance characteristics, because the formation ages of sapphires from various well-known deposits are different. Although the sapphires from Muling are much older than the oldest human civilization, they are among the youngest sapphire deposits known to date (e.g., Madagascar, Sri Lanka, Myanmar, and North America). Therefore, the U-Pb age of zircon inclusions is an efficient indicator in separating similar-looking sapphires from various deposits.
Once more age information on sapphires is collected, a totally new system of provenance identification will be established. It is also helpful for further understanding the genesis of gemstones and instructing people on the rational utilization of resources.
REFERENCES
Ling Y.Y., Zhang J.J., Liu K., Ge M.H., Wang M., Wang J.M. (2017) Geochemistry, geochronology, and tectonic setting of Early Cretaceous volcanic rocks in the northern segment of the Tan–Lu Fault region, northeast China. Journal of Asian Earth Sciences, Vol. 144, pp. 303–322, http://dx.doi.org/10.1016/j.jseaes.2016.12.025
Liu Y, Lu R. (2016) Gem News International: Ruby and sapphire from Muling, China. G&G, Vol. 52, No. 1, pp. 98–100.
Wang X.K., Qiu S.W., Song C.C., Kulakov A., Tashchi S., Myasnikov E. (2001) Cenozoic volcanism and geothermal resources in northeast China. Chinese Geographical Science, Vol. 11, No. 2, pp. 150–154, http://dx.doi.org/10.1007/s11769-001-0035-z



