Diamond Identification

Complex Charge Transfer in Chameleon Diamonds: A Model of the Color-Change Process
James E. Butler1,2, Keal S. Byrne2, Wuyi Wang3, and Jeffrey E. Post2
1Cubic Carbon Ceramics, Washington, DC
2Department of Mineral Sciences, Smithsonian Institution, Washington, DC
3GIA, New York
A group of natural diamonds known as chameleon diamonds change color from green to yellow based on their exposure to light and heat. These diamonds also emit long-lived phosphorescence after UV excitation. We have observed the optical response of these diamonds to optical and thermal excitation and developed a model to explain the observed phenomena. A principal element of the model is the proposal of an acceptor state (figure 1), which should be observable in the near-infrared (NIR) region. Subsequently, we have observed the NIR absorption to this acceptor state, supporting our model of charge-transfer processes in these diamonds.
Melee Diamonds: Metal Defects and Treated Color
Shoko Odake
GIA, Tokyo
Gem-quality laboratory-grown diamonds are manufactured in large quantities. With frequent reports of the mixing of melee-sized synthetic diamonds with natural stones, demand for melee diamond screening is increasing. During melee diamond screening at GIA’s Tokyo lab, two notable types of samples with uncommon characteristics have been found.
- Natural melee diamonds with silicon and nickel defects. Luminescence peaks derived from Si- and Ni-related defects are often observed in colorless melee grown by the HPHT method. The silicon-related defect, once considered proof of CVD-grown diamond, is now known to exist naturally as well (Breeding and Wang, 2008). Several colorless melee diamonds having both silicon- and nickel-related emissions have been found in GIA’s Tokyo lab; olivine inclusions were found in one of these samples. Spectroscopic and gemological features confirmed that the samples were grown in nature.
- Irradiated laboratory-grown diamond melee found among irradiated natural melee diamonds. Several thousand greenish blue melee diamonds have been submitted by various clients to the Tokyo lab for testing. Each diamond’s color was attributed to a strong GR1 defect caused by irradiation treatment. Fourier-transform infrared (FTIR), photoluminescence (PL), and DiamondView analysis revealed that most of them were irradiated natural diamonds. Eight were irradiated CVD-grown diamonds, and one was an irradiated HPHT-grown specimen. The infrared spectrum of all the CVD samples showed a peak at 3123 cm–1, while their PL spectrum showed a doublet peak at 596/597 nm. Those peaks are specific to as-grown CVD diamonds, as annealing removes the peaks. From their spectra, these CVD specimens were considered irradiated without pre-annealing.
REFERENCE
Breeding C.M., Wang W. (2008) Occurrence of the Si-V defect center in natural colorless gem diamonds. Diamond and Related Materials, Vol. 17, pp. 1335–1344, http://dx.doi.org/10.1016/j.diamond.2008.01.075
Nitrogen in CVD-Grown Diamond
Alexander M. Zaitsev1,2, Kyaw Soe Moe2, and Wuyi Wang2
1College of Staten Island, City University of New York
2GIA, New York
In diamond grown by the CVD method, nitrogen behaves differently than it does in natural and HPHT-grown diamond. The most striking peculiarities are low efficiency of doping, formation of unique optical centers over a wide spectral range from the ultraviolet (UV) to the IR regions, and formation of unusual defects related to aggregated nitrogen. In order to gain a better insight into this problem, several nitrogen-doped specimens grown in GIA’s CVD diamond lab and a few commercial yellow CVD-grown diamonds have been studied in their as-grown (as-received) state and after electron irradiation and annealing at temperatures up to 1900°C (low-pressure, high-temperature treatment).
We found that the brightest pink color of electron-irradiated nitrogen-doped CVD-grown diamond is produced by the NV– center after annealing at temperatures of about 1000°C. Annealing at temperatures over 1600°C destroys the irradiation-induced pink color (figure 1).
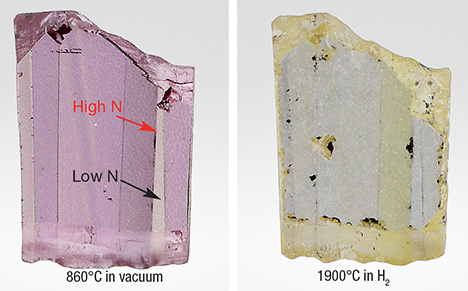
The most prominent optical centers in the IR spectral region (figure 2, left) produced absorptions at 2828, 2874, 2906, 2949, 3031, 3107, 3123, and 3310 cm–1 (latter two not shown). These are ascribed to nitrogen-hydrogen complexes. Two characteristic absorption features at 1293 and 1341 cm–1 (figure 2, right) are unique to CVD diamond. They are tentatively ascribed to a modified form of nitrogen A-aggregates.

In the visible and NIR spectral ranges, characteristic nitrogen-related centers have zero-phonon lines (ZPLs) at 457, 462, 489, 498, 647, 722.5, 852.5, 865.5, 868.5, 908, 921.5, and 924.5 nm. The 489 nm feature is a major color center of electron-irradiated, nitrogen-doped CVD-grown diamond. This center, together with the GR1 center, is responsible for the green color in this material.
An assumption is made that N atoms may form clusters in highly nitrogen-doped CVD-grown diamonds. These clusters may result in broad-band luminescence at wavelengths of 360, 390, 535, and 720 nm and a strong broadening of the ZPLs of many optical centers.
Steps in Screening and ID of Laboratory-Grown Diamonds with Synthetic Diamond ID Kit
Branko Deljanin1 and John Chapman2
1CGL-GRS Swiss Canadian Gemlab, Vancouver, Canada
2Gemetrix, Perth, Western Australia
Laboratory-grown diamonds are created using either high-pressure, high-temperature (HPHT) or chemical vapor deposition (CVD). With the influx of manmade diamonds on the market over the past few years, instrument producers and labs have launched screening and detection instruments to help dealers and jewelers spot HPHT- or CVD-grown specimens.
Most standard instruments are inaccurate testers or just type I and type II screening devices that do not give a definite answer about diamond genesis. Over the last four annual Mediterranean Gemmological and Jewellery Conferences and more than 30 workshops given in 17 countries, we have assembled a portable new Synthetic Diamond Identification Kit. The kit comprises two portable instruments and two booklets:
- A PL inspector (mini UV lamp with magnifier) to inspect laboratory-grown, treated, and natural diamonds using long- and short-wave fluorescence and phosphorescence
- A 2017 handbook with images and explanation of long- and short-wave reactions of diamonds of all types
- A mini foldable polariscope with portable light to separate natural diamonds using characteristic birefringence patterns from HPHT and CVD diamonds
- A 2010 handbook with images and explanations of cross-polarized filter reactions of diamonds of all types
The combination of this kit with professional training could identify all HPHT-grown diamonds and most CVD-grown diamonds on the market, loose or mounted. Also available are melee and jewelry inspectors consisting of larger UV lamps with magnifiers designed for identification of small loose or mounted diamonds.
Different diamond types and subtypes can exhibit different birefringence under cross-polarized filters. A clear majority of natural diamonds exhibit some degree of internal strain, with type II natural diamonds showing a weak “tatami” pattern. HPHT-grown diamonds are free of such strain, and CVD-grown diamonds show mostly coarse columnar patterns.
Most natural diamonds have a strong reaction to long-wave UV; this reaction is usually weaker (mostly blue) at shorter wavelengths. Laboratory-grown diamonds generally exhibit more intense fluorescence with short-wave UV compared to long-wave UV, with a chalky coloring tinged with green or yellow. Most HPHT-grown diamonds also phosphoresce. If a diamond is free of inclusions, fluorescence is a reliable screening test to flag suspicious stones that should be further checked under cross-polarized filters (figure 1).

In the case of some rare near-colorless clean CVD-grown diamonds that do not show fluorescence or have a birefringence pattern that is coarse but resembling tatami in type IIa and weak patterns in natural Ia diamonds, additional tests using advanced spectroscopy and strong short-wave UV light to observe growth patterns are needed to confirm diamond genesis.



