Boron Carbide: A New Imitation of Black Diamond


Figure 2. Under reflected light, the boron carbide specimen displayed a dense, fine granular structure typically associated with aggregates such as ceramics. Photomicrograph by Gagan Choudhary; magnified 48×.
Due to the prevalence of black synthetic moissanite in the marketplace and gem laboratories, we immediately checked for the typical aggregate structure under the microscope. Although a granular pattern was distinctly visible in reflected light (figure 2), it was much denser and finer than the kind usually seen in black synthetic moissanite. This raised uncertainty, so additional gemological tests were performed. The specimen had an over-the-limit RI, an SG of 2.43, and a hardness above 9 on the Mohs scale. The low SG value ruled out the possibility of synthetic moissanite (3.22), but otherwise offered no clues as to the specimen’s identity.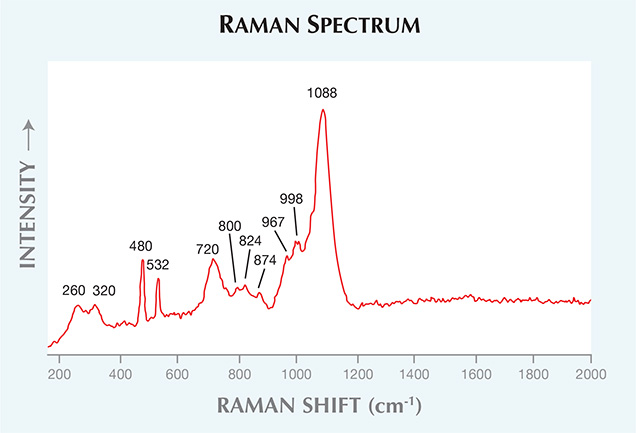
Figure 3. The boron carbide’s Raman spectrum showed major peaks at approximately 260, 320, 480, 532, 720, and 1088 cm–1.
Qualitative EDXRF analysis revealed Fe, with traces of Si and K. Raman spectra taken from several points using 532 nm laser excitation showed major peaks at approximately 260, 320, 480, 532, 720, and 1088 cm–1, with smaller peaks at about 800, 824, 874, 967, and 998 cm–1 (figure 3). This combination of features did not match anything in our database, but an extensive literature search revealed an exact match with the Raman spectra of boron carbide (V. Domnich et al., “Boron carbide: Structure, properties, and stability under stress,” Journal of the American Ceramic Society, Vol. 94, No. 11, 2011, pp. 3605–3628, http://dx.doi.org/10.1111/j.1551-2916.2011.04865.x).Boron carbide is an advanced ceramic material with high hardness, low density, thermal stability, and extreme abrasion resistance, making it suitable for nuclear, military, and aerospace applications. The material is typically produced by reacting and fusing carbon with boric oxide (B2O3) in an electric arc furnace, followed by a sintering process of hot pressing in graphite dies under a vacuum or argon atmosphere at temperatures of 1900–2200°C and pressures of 0.02–0.04 GPa for 15–45 minutes (Singhal and Singh, “Sintering of boron carbide under high pressures and temperatures,” Indian Journal of Engineering and Materials Sciences, Vol. 13, April 2006, pp. 129–134).
Although boron carbide is common in engineering and materials science—used as scratch-resistant coating and plating in tank armor, bulletproof vests, and padlocks—this was our first encounter with it as a diamond simulant. Our literature search (by Mr. Sandeep Vijay, a staff gemologist) did not reveal any gemological uses of this ceramic product. Distinguishing boron carbide from black diamond or synthetic moissanite was straightforward on the basis of SG, but Raman spectra and reference data were needed for identification. The possibility of market penetration cannot be ruled out.



