Dumortierite-Quartz Rock Presented as Sapphire

Gemological testing gave a vague spot RI around 1.55 and a hydrostatic SG of 2.99.The stone fluoresced strong blue to short-wave UV and was inert to long-wave UV. It showed no reaction when viewed with the Chelsea color filter and no distinct absorptions using the desk-model spectroscope, reactions that would preclude the possibility of dyeing. The RI was consistent with quartz, but the SG value ruled out that possibility. Because of the curved surface and opacity of the cabochon, we asked the client to provide a specimen with a flat polish, enabling us to properly study the material. The client also provided a thin slice of the material (figure 15, right), which allowed light to pass through easily. The properties of the slice were similar to those recorded for the cabochon. Two RI readings were obtained while slightly shifting the slice, however. Although the readings were not clear, shadow edges were seen at about 1.54 and 1.68.
Initial microscopic observations of the slice with strong transmitted light suggested blue color concentrations along the boundaries of white to colorless grains, as seen in dyed materials (figure 2, below). Yet the interstitial areas were wider than those of dyed quartzite or any other dyed substance. On further examination of the slice, inhomogeneous blue and white granular texture became evident, where some blue grains were also visible. Reflected light revealed a clear difference between the blue and whitish portions (figure 16, right). The sharp-edged whitish irregular grains, which appear gray in the image, seemed to be embedded in the blue matrix. Also, the white portion had a much duller luster than the blue one. These features suggested that the blue color was indeed natural and that the uneven coloration was not the result of dyeing, but rather a mixture of white and blue materials.
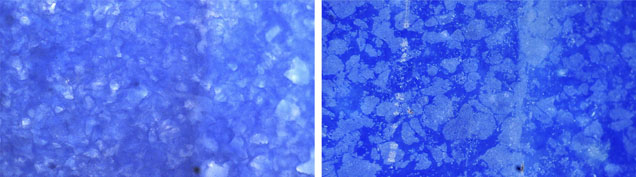
Figure 2. Uneven blue and colorless areas (left) suggested color concentrations associated with a dyed specimen, but reflected light (right) revealed a clear difference between the blue and whitish portions. The sharp-edged whitish irregular grains (which appear gray in the image) seemed to be embedded in the blue matrix. The white mineral was identified as quartz and the blue mineral as dumortierite. Also note the difference in the luster of two areas, although both minerals have similar hardness. Photomicrographs by G. Choudhary; magnified 32×.
UV-Vis-NIR spectroscopy of the slice in the 200–2000 nm region showed broad absorption bands (figure 3) centered at ~290 and ~610 nm, which are assigned to the Fe2+-Ti4+ charge transfer (see Platonov et al., “Fe2+-Ti4+ charge-transfer in dumortierite,” European Journal of Mineralogy, Vol. 12, No. 3, pp. 521–528). This was confirmed by EDXRF analysis, which detected the presence of Fe and Ti along with Al, Si, Ca, and As. In the DiamondView, the blue portions gave strong blue reactions while the white grains remained inert (figure 4).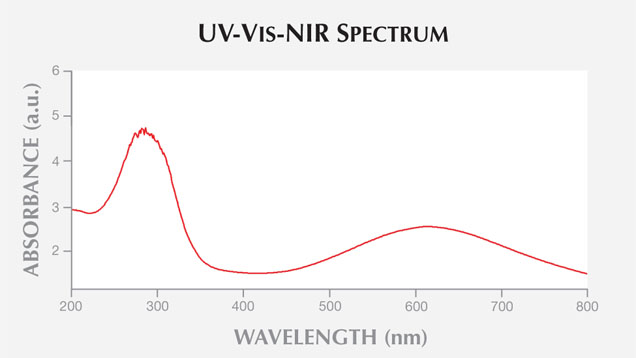
Figure 3. The slice’s UV-Vis-NIR spectrum in the 200–2000 nm region showed broad absorption bands centered at ~290 and ~610 nm, which are assigned to Fe2+-Ti4+ charge transfer and associated with dumortierite.
Raman analysis using a 532 nm laser confirmed the blue and white minerals as dumortierite and quartz, respectively. The blue durmortierite revealed major peaks in the 200–2000 cm–1 region at ~206, 290, 396, 446, 506, 844, 945, and 1068 cm–1, while the white quartz showed major peaks at ~206, 263, 353, 464, 807, 1080, and 1157 cm–1.These peaks are consistent with those reported for dumortierite and quartz in the RRUFF database. Furthermore, the SG value of 2.99 suggested a composition of approximately 54.64% quartz and 45.36% dumortierite (assuming an SG of 2.65 for pure quartz and 3.40 for dumortierite, while disregarding other accessory minerals).
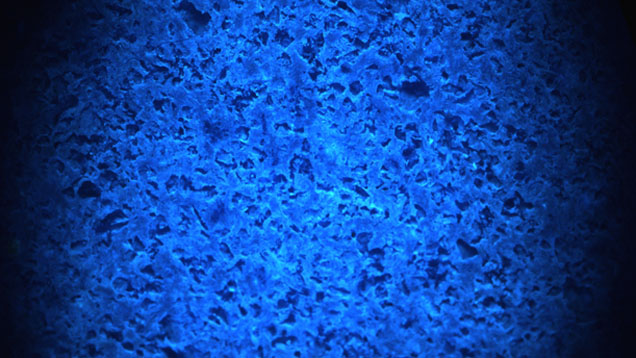
Figure 4. DiamondView imaging revealed strong blue fluorescence in the dumortierite portion of the slice, while the quartz grains appeared dark and remained inert.
Identification of this specimen would have been difficult in the absence of Raman analysis, though UV reactions and a vague RI shadow edge offered some clues. For several years dumortierite has been known to occur with quartz as rock and massive forms, but it is not routinely seen in the trade, perhaps due to lack of awareness or because it is being misrepresented, as in this case. The client had no information on the origin of these specimens, which were purchased on the local market, but dumortierite in quartz has been reported from India (R. Webster, Gems, 5th ed., revised by P. G. Read, Butterworth-Heinemann, Oxford, UK, 1994).


