Pink Sapphire Filled with Bismuth-Based Glass

Recently, GIA’s Bangkok laboratory received a 2.77 ct light pink sapphire (figure 1) for an identification report. The sample was a semi-transparent cabochon with natural inclusion features: silk needles and particles, twinned planes, and thin films. Also observed throughout the stone were numerous gas bubbles of various shapes and sizes that were trapped along surface-reaching fractures and cavities (figure 2). These indicate the presence of glass filling. However, no flash effect was evident in the filled fractures. The stone’s refractive index of 1.76 and UV fluorescence reaction (medium red to long-wave and weak red to short-wave) were consistent with corundum. Its specific gravity of 3.87 was lower than that of corundum and possibly a result of the filler. Due to the sapphire’s very light color, its visible-range absorption spectrum was difficult to observe.

Qualitative chemical analysis using energy-dispersive X-ray fluorescence (EDXRF) spectroscopy showed the presence of bismuth in addition to the expected Al, Cr, Fe, and Ga. No lead was detected in the sample, even with the aid of the thick palladium filter. The chemical composition of the filler indicated that the stone had undergone fracture filling using bismuth-based glass (“Ruby treatments – revisited, with Mozambique’s perspective,” Lab Information Circular, Gem Testing Laboratory, Vol. 63, December 2011). Due to the large difference in refractive indices between the bismuth-based glass filled area and the surrounding corundum, this treatment was easier to visually detect than lead-glass filling (figure 3).
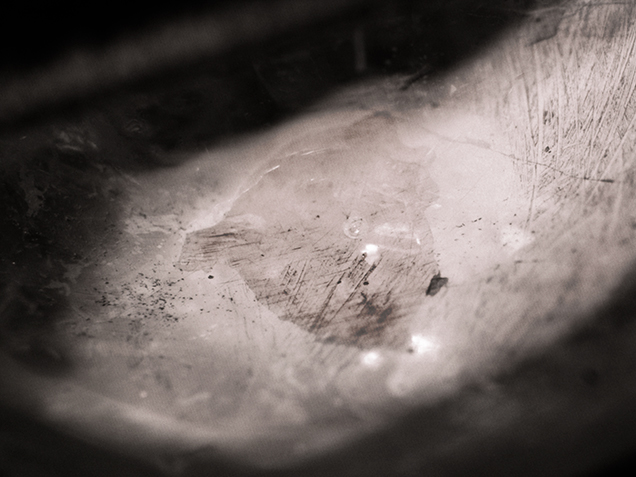
Fracture filling with lead glass and non-lead glass has been widely used in low-grade ruby (S.F. McClure et al., “Identification and durability of lead glass–filled rubies,” Spring 2006 G&G, pp. 22–34). Pink sapphires such as this 2.77 ct stone are less commonly encountered in the laboratory for examination and identification.



