Bead-Cultured and Non-Bead-Cultured Pearls from Lombok, Indonesia

INTRODUCTION
Cultured pearls are harvested from wild and hatchery-reared mollusks of various Pinctada species in many parts of the world. Today, most of the oysters that form these cultured pearls are raised in hatcheries, and only a few pearl farms still operate using wild oysters. On the Indonesian island of Lombok, the farming procedures follow the industry norm. Some of the authors had the opportunity to visit a Lombok farm (figure 1) in early 2013 to observe the opening and, in some cases, the reseeding/renucleation of numerous P. maxima oysters (predominantly gold-lipped). During the one-day visit, we saw a limited number of bead-cultured pearls and even fewer non-bead-cultured pearls (“keshi”) recovered from oysters that were opened by the farm owners for the benefit of this research trip. Photos of the recovery and the samples collected, as well as the results of their subsequent analysis, are shared so that information on samples of known origin is available in the gemological literature. Analysis of known samples recovered and recorded in this way removes all doubt and gives unequivocal data that can be used with full confidence.
TRIP AND FARM DETAILS
The visit was arranged by author JB, who liaised with Mohamad Irfan Bin Ansori and Wiwi Aswiana, the directors of Jakarta-based Maisya Jewellery. Through their relationship with the owners of the farm, it was possible to visit the operation within a matter of days. The farm itself is located at 8˚24′46.33′′ S 116˚42′45.91′′ E (figure 2), off the northeast coast of Lombok, one of the 13,466 islands that make up Indonesia. At an elevation of 3,726 meters, Mount Rinjani is the highest point on Lombok. This active volcano is visible from the farm, though cloud cover obscured the summit on the day of our visit.
The trip from Mataram, Lombok’s largest city, took approximately two hours by car—not because of the distance but rather the twisty route through the countryside. The beauty of the sea and the farm’s unspoiled environment, both important factors when trying to coax P. maxima oysters to produce beautiful pearls, were immediately obvious. The boundaries of the facility were easily within view. Some of the larger Indonesian farms extend for several miles, so this one could be classified as a small-scale operation. This did not mean that the operation was inferior in any respect, and the authors were impressed by the hatchery in particular.
The most important building on the site was the hatchery, a rather nondescript white structure where all the mollusks used for culturing are raised. While some wild mollusks may have been used to breed offspring for the farm’s initial harvests, this does not appear to be the case today. Culturing from farm-raised mollusks seems to be a trend in farms around the world, with the exception of Australia, where a high percentage of wild shells are still used (Marine Stewardship Council, 2015).
During the tour, our hosts were very open and cooperative and allowed us to enter the hatchery and take photographs. The dimly lit interior housed numerous rows of concrete tanks with larvae and spat at various stages of development. In another section of the hatchery, we saw two additional rows of transparent plastic tanks covered with black material to protect the occupants. Finally, in another part of the building we met a young Indonesian biologist who was responsible for ensuring that all the oyster larvae and spat were healthy and eventually able to produce pearls. Shelves in one air-conditioned room of the hatchery were lined with several containers of green, yellow, orange, and colorless liquids (figure 3), a common sight at farm operations today (Cartier et al., 2012). The colored ones contained the various types of phytoplankton at different life stages that were being grown to feed the spat until they were old enough to be moved to the open sea. The role of the marine biologist we met, and others like him throughout pearl farms globally, cannot be underestimated. They are entrusted with making sure that future generations of mollusks thrive and reach an age and size where they can be operated on and produce pearls. Without the knowledge and care of these specialists, the whole concept of pearl farming would not exist.

MATERIALS AND METHODS
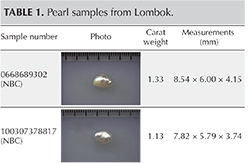
The lead author recovered three non-bead-cultured (NBC) pearls directly from the gonad region of their host mollusks and assisted with the removal of some of the nine bead-cultured pearls (including the one shown in table 1) recovered from other mollusks. While some of the bead-cultured pearls were removed from pearl sacs in the gonad region, like the three NBC pearls (table 1), a few were found loose in the hinge area after opening, as they must have fallen out of their sacs during the process. One “seed” pearl was recovered by the lead author during the opening of a shell that also produced a bead-cultured pearl. However, the exact area where the pearl formed within the shell was not clear since it was only found when the organs were being checked for pearls. Those who have searched for pearls in the soft organs of a mollusk know that it is not always easy to find the smaller pearls in field conditions, so the exact formation location of such pearls is rarely recorded. They are usually found during the final step, when someone meticulously searches the organs. One other shell opened for us was barren. The bead nucleus must have been ejected shortly after insertion, and the mantle implant did not produce a sac in which a pearl could form.
The pearls’ internal structures were examined using a Matrix-FocalSpot XT-3 Series real-time X-ray (RTX) machine (90 kV and 5 micron excitation) fitted with a Toshiba image intensifier. A ProCon CT-Mini model X-ray computed microtomography (μ-CT) unit fitted with a Thermo Fisher 8W/90 kV X-ray tube and a Hamamatsu flat-panel sensor detector was used specifically for the three non-bead-cultured pearls and one “seed” pearl. Since all the pearls were white, no Raman or ultraviolet/visible/near-infrared (UV-Vis-NIR) spectra were obtained; these are usually collected on colored pearls. Nor did we record the surface structures or ultraviolet reactions (UV spectroscopy and DiamondView imaging), as the main purpose of this work was to document the internal structures. Even though the pearls were collected from a saltwater environment, their composition was still checked for reference purposes using a Thermo XSeries II laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) system equipped with an attached New Wave Research UP-213 nm laser. Microanalytical carbonate standards MACS-1 and MACS-3 from the United States Geological Survey (USGS) were used.
OBTAINING THE SAMPLES
The main purpose of the trip was to observe Pinctada maxima samples being opened in field conditions so we could record the positions of the pearls recovered. We ultimately wanted to obtain known examples of non-bead-cultured pearls for further examination in GIA’s Bangkok laboratory, as these are the types of pearls most likely to pose identification challenges. Building a reference database of pearls with structures associated with a known provenance is the surest way of solving complex identification issues that may arise. Even though it was not officially harvest time when we visited, our hosts kindly agreed to open a selection of shells at random to see what they contained (figure 4).

Since the formation of pearls is a purely biological mechanism, it is only through periodic X-ray checks that farms are able to see whether their painstaking work on the mollusks will be rewarded with a pearl. When each mollusk is opened, it is in the hands of fate whether a pearl has actually formed, and whether it is of sufficiently high quality to be considered a gem. Many of the pearls produced on farms around the world are misshapen, blemished, or dull. Because we opened only 13 mollusks, the chances of finding a pearl of any worth were extremely low. The likelihood of finding a non-bead-cultured pearl was even more remote, since the farmers’ intent is to produce bead-cultured pearls and not the accidental non-bead-cultured pearls (“keshi”) that sometimes occur when beads are ejected from a pearl sac, among other circumstances. While “keshi” might not be the scientifically correct name (Hänni, 2006), the term has become synonymous with pearls that are accidentally formed as a result of bead-culturing in all types of commercially farmed nacreous mollusks (Pteriidae family especially).
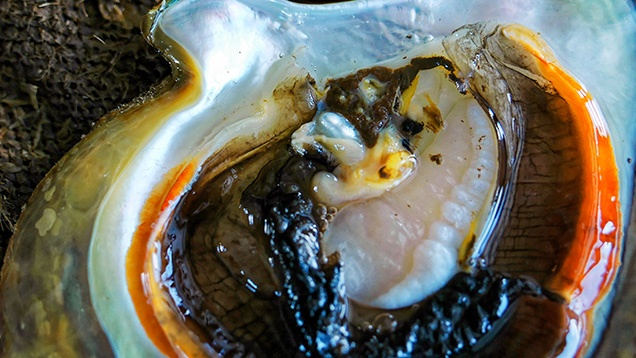
Fortune was on our side, as three of the shells opened for us contained a non-bead-cultured pearl in the gonad area. Cultured pearls will only form where the graft is located; nacre-forming epithelial cells are only found in the gonad after the cells have been inserted there during the nucleating process. In unoperated mollusks, these cells are situated only in the mantle tissue that lines both halves of the shell. As with most non-bead-cultured pearls, the samples recovered during this field trip were baroque and exhibited a high luster.
The farm owners were kind enough to allow us to retain the pearls as well as the shells from which they were removed. This allowed us to compare the platy formation of the nacreous pearls and their hosts.
INTERNAL STRUCTURES
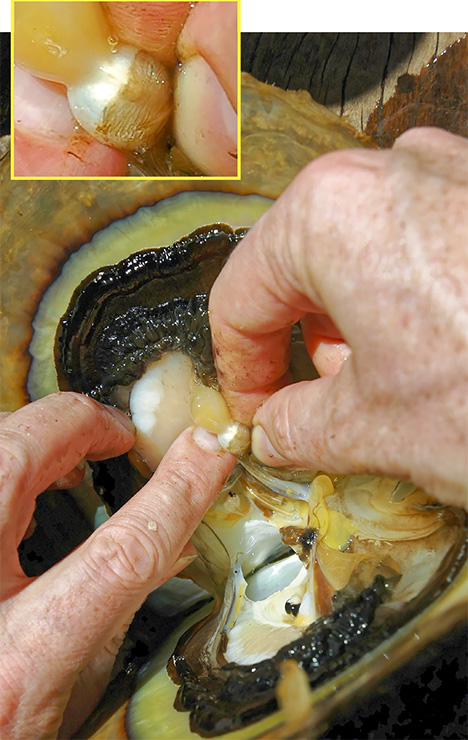
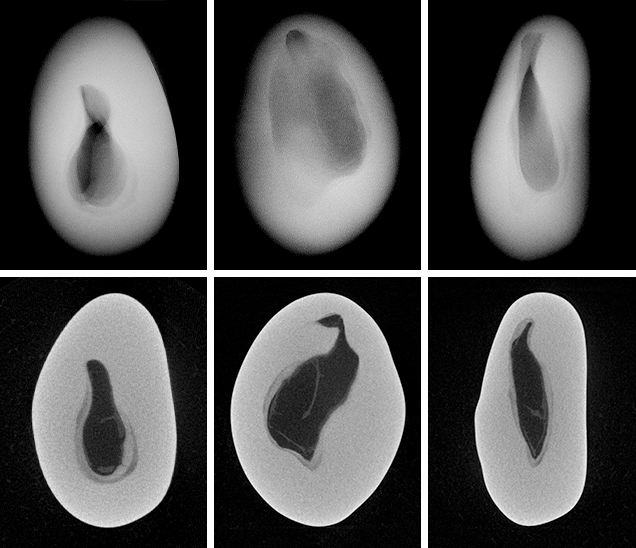
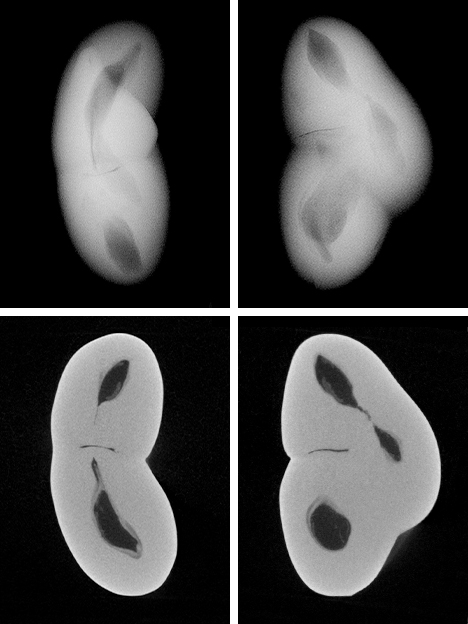
Since the purpose of the field trip was to source non-bead-cultured pearls and record their positions in the mollusks, we only detailed the internal features of some of the bead-cultured pearls recovered, one of which is shown being removed from its host in figure 5. Only one (sample no. 100307378805) is included as a representative sample of the typical structure found in such pearls (RTX images only). The one chosen happens to be orientated in a way that clearly shows the banding within the shell nucleus (figure 6) used to instigate the pearl’s formation. The banding is visible because of its alignment within the bead nucleus relative to the detector and the X-rays generated from the RTX unit. In some examples, the banding is very distinct in an optimal orientation.
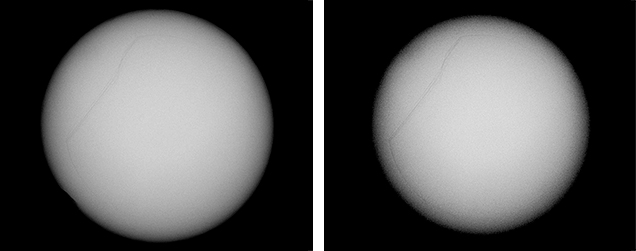
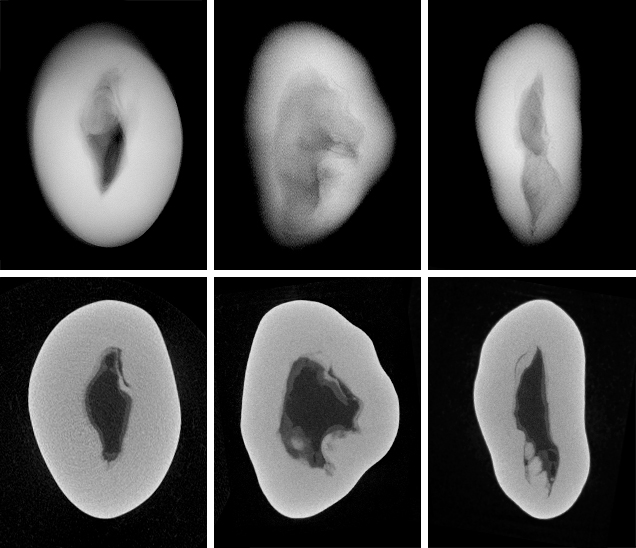
The three NBC pearls (figures 7, 9, and 11) showed structures consisting of void features in a nacreous body that lacked distinct concentric rings or surrounding arcs (figures 8 and 10). However, a conchiolin-rich deposit that followed the outline and hence the shape of the void was present around the voids in all three pearls to varying degrees. This deposition, though not always observed in NBC pearls, is nonetheless a common feature, based on the lead author’s experience. Two of the pearls showed one clear central irregular void feature, while the third showed pairs of voids in two separate growth areas. The most common feature of saltwater NBC pearls is a single void (Sturman, 2009). Yet, as the latter example (figure 12) clearly illustrates, two or more voids may also be encountered. Most of the saltwater NBC pearls tested at GIA locations globally show at least one fairly large void or several void features, depending on the shape being examined. The tighter forms showing other features such as linear structures or more natural-looking structures are much less likely to be encountered in a gemological laboratory. But these are the types that can create considerable differences of opinion, which is why collecting known samples on our trip was so important.
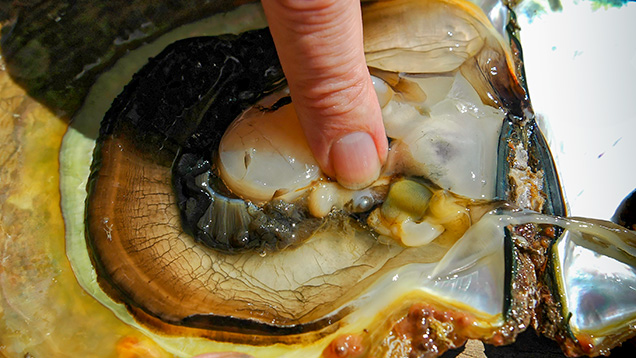

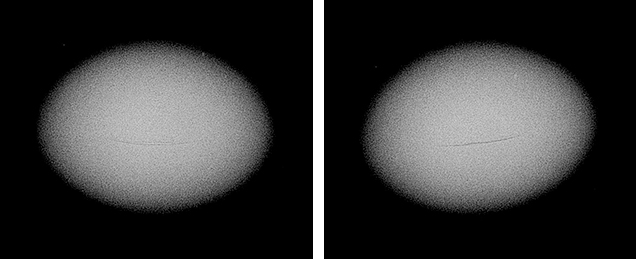
Seed pearls like the one found on this trip (figure 13) present their own identification challenges, owing to their small size. Although some show clear natural or NBC structures that prove their origin, many have tight or limited structures that may lead to differences of opinion on their identity. Since the seed pearl we found showed only one weak arc (figure 14), it might be considered more natural by some gemologists. Yet its true classification would be debatable given that it was found in a hatchery shell but likely formed in a pearl sac that was not initiated by humans.

CHEMICAL COMPOSITION
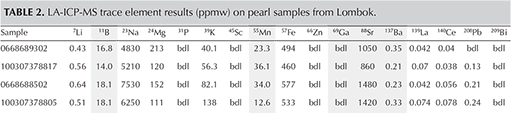
Since the pearls are of known saltwater origin, their chemical composition is included purely for academic interest. Work on saltwater pearls from P. maxima has already been documented (Scarratt et al., 2012), and our results support the findings of this previous work. The manganese content of saltwater pearls is generally accepted to be much lower than that of freshwater pearls; strontium, gallium, barium, and boron levels also differ between saltwater and freshwater mollusks. Table 2 shows the concentration (parts per million) of these and other elements in the samples from this study.
CONCLUSIONS
The three indisputably non-bead-cultured pearls described here, which were removed by the lead author, are just a minute selection of the pearls removed by GIA research staff from shells taken from the open ocean (according to permitted quotas for mother-of-pearl recovery) or from farms around the world. While the structures did not prove to be unusual in any way, and hence fall within those expected for accidentally formed “keshi” NBC pearls, the recording of their structures should assist those in the trade who deal in or conduct research on examples of such pearls from known sources.
Trips to other farms and natural pearl sources worldwide to recover pearls of known origin (figure 15) have helped establish a GIA database of structures, and more visits are planned over the coming months and years. Only by continuing to work with farmers and shell seekers can such a database be accomplished, and only with their cooperation is it possible to accumulate such important samples and knowledge. For that, GIA and the trade cannot be appreciative enough.













.jpg)


